- LOGIN
- MemberShip
- 2025-12-25 04:43:43
- NHI billing soars and brings down Xeljanz price by 9%
- by Eo, Yun-Ho | translator Byun Kyung A | 2019-11-30 05:50:42
Starting from next month, reimbursed price of Pfizer Korea’s Janus kinase (JAK) inhibitor Xeljanz (5 mg) is to be lowered by 9 percent.
The treatment is now included as subject for price-volume agreement (PVA), because the actual insurance billing amount was surged by 30 percent than the initially estimated amount.
Also for Allergan Korea’s Pred Forte Eye Drop, its reimbursed price would be brought down by 1.7 percent as actual billing amount of the same class items was surged by 30% than the estimated amount.
Human growth hormone injection Eutropin by LG Chem is now a subject for preliminary price reduction by 3.3 percent after securing an additional indication.
According to pharmaceutical industry source on Nov.
22, the government is preparing an updated list of reimbursed drug price and upper limit price with the said changes.
When the list is finalized, the updated prices would be in effect from Dec.
1.

Its price would be brought down by 21.2 percent, from 16,650 won to 13,112 won.
The treatment’s price is to be dropped when the discretionary price adjustment ends the weighted pricing period.
The government provides weighted pricing at 70 percent of the original’s price to the first generic to get listed, which it lasts for a year.
But if there are less than three manufacturers with the equivalent class of generics after a year, the weighted pricing can be maintained until the fourth one is listed.
After negotiating with National Health Insurance Service (NHIS), three items now have PVA in type Ga (가) and Na (나).
Their listed price would also fall and it would be reflected from next month.
Categorized as type Ga, Allergan Korea’s Pred Forte Eye Drop was initially listed after pricing negotiation but actual NHI billing amount of other items in the same class surpassed the estimated amount by 30 percent since the point of negotiation.
Pricing of the eye drop in 50 mg/5 mL vial and in 0.1 g/10 mL vial are to go down from 2,569 won to 2,525 won, and 5,138 won to 5,050 won, respectively, by 1.7 percent.
Although not categorized as type Ga, Xeljanz tablet was categorized by PVA type Na after being listed for four years.
The overall billing amount of other items in the same class has gone over the estimated amount by 30 percent, and the tablet was then included among the group of items in the same class with adjusted upper limit price.
Xeljanz met the criteria of PVA type NA, which recognizes an item with actual insurance billing amount increased from the year before by either 60 or 10 percent, and by over 5 billion won.
According to the negotiation result, the treatment price is to be reduced by 8.9 percent from 12,992 won to 11,836 won.
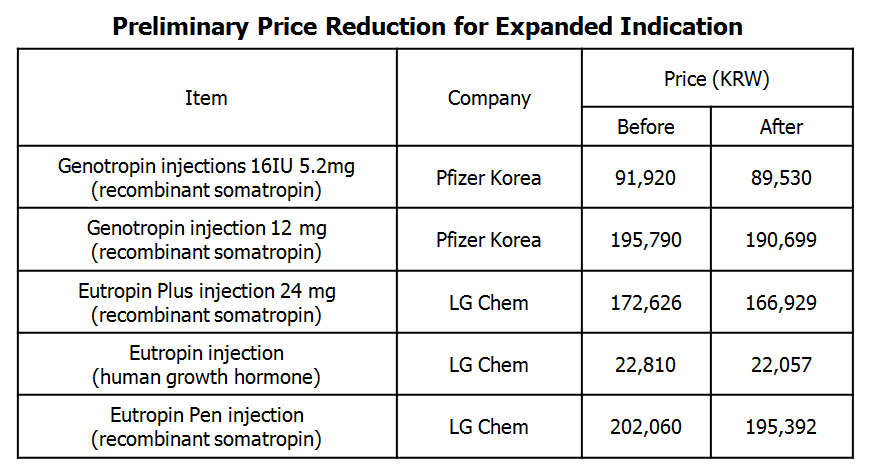
Five items, including Pfizer’s Genotropin injections (16IU and 12 mg), LG Chem’s Eutropin Plus injection (24 mg), Eutropin injection and Eutropin Pen, are subject for preliminary drug price reduction with expanded indication.
These drug items are to reduce prices beforehand considering additional estimated billing amount and increase rate based on expanded indications.
Reimbursed prices of Genotropin injection 16IU and 12 mg vial are to get reduced by 2.6 percent, from 91,920 won to 89,530 won and 195,790 won to 190,699 won, respectively.
All Eutropin injection prices are to get lowered by 3.3 percent each.
Prices of Eutorpin Plus injection (24 mg), Eutropin injection, and Eutropin Pen are to be lowered from 172,626 won to 166,929 won, from 22,810 won to 22,057 won, and from 202,060 won to 195,392 won, respectively.
Another item’s price was raised after a pricing negotiation with NHIS as its request for an adjustment on upper limit price was accepted.
Access Pharma’s Tuberculin PPD RT 23 SSI/APC is a substance used for tuberculosis skin test, and the company submitted an application for upper limit price increase due to import price raised by privatization of its manufacturer.
The substance price would be raised from 19,225 won to 24,000 won.
Chong Kun Dang’s Raparobell tablet (2 mg) went through a discretionary adjustment last month as expected when the original Rapamune tablet’s price was voluntarily reduced in March, 2016.
The reduction was made after negotiating with NHIS, as generics were getting listed.
Raprobell’s current price, 3,018 won would be bumped up to 4,311 won.
With the first generic getting listed, the price of Rapamune tablet (2 mg) was adjusted down to 70 percent at 4,438 won.
Accordingly, the generic tablet’s price was increased up to 68 percent of the first-in class item’s price.
Other three items’ prices are to be lowered by their companies, voluntarily.
Il Yang Pharmaceutical is lowering Il Yang Choline Alfoscerate capsule price by 8.2 percent, from 523 won to 480 won.
Whereas Nelson Korea is to reduce price of Nelson Donepezil tablet (5 mg) by 53.8 percent, from 1,300 won to 600 won, and Jinyang Pharm to reduce Tacromin capsule price by 16.9 percent, from 3,630 won to 3,015 won.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









