- LOGIN
- MemberShip
- 2025-12-25 04:43:39
- First year of Rare Disease Support Scheme
- by Eo, Yun-Ho | translator | 2019-11-04 08:13:12

‘Rare disease’ is not a specific categorization, but rather it is designated based on frequency of diagnosis.
Korea defines ‘rare disease’ as a disease diagnosed to less than 20,000 people.
With small patient size and lack of drug, these diseases are in dire need of new drug.
But the voices of small handful of patients are easily lost in the air.
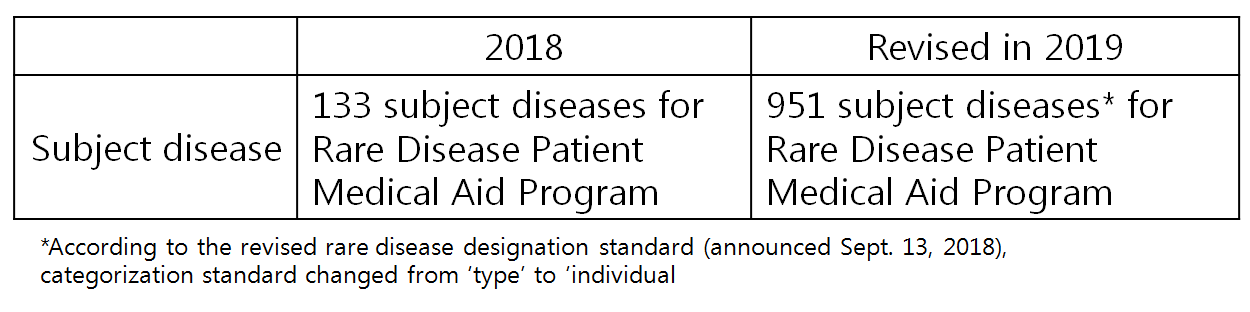
At the moment in Korea, 951 diseases are designated as subject for Rare Disease Medical Aid Program, in which 927 of them are eligible for special case benefit.
The total of 927 consists of 827 rare diseases as defined by the National Health Insurance special case copayment benefit system, and about 100 more added, as of August 2017, by a rare disease survey reflecting opinions of patients and their families, patient advocacy group, and medical experts.
Previously, the government did not have a government-managed rare disease list without sufficient legal basis to back it up.
So the size of rare disease patients was estimated according to the special case benefit subject list.
Rare and chronic diseases were confused and defined as one keeping rare disease related policy making and researches limited.
But growing voices criticized government for neglecting rare disease, and finally the Rare Disease Management Act was enacted in 2016.
Since then, the first Rare Disease Management Plan was established, and in September 2018, the Rare Disease Patient Support Scheme was first implemented as more demanded for state-level rare disease patient support like disease management, treatment and prevention.

The health authority applied special case benefit on the new rare diseases and expanded eligible disease for low-income patient medical aid.
Including the newly designated 100 rare disease, now about 1,800 patients receive special case benefit, annually.
Special case benefit system for rare disease patient started from applying 20 percent of copayment rate on artificial kidney dialysis or continuous ambulatory peritoneal dialysis for chronic kidney failure patients, and the benefit continued to expand on hemophilia, Gaucher’s disease, leukemia and cancer patients.
But some undiagnosed rare disease patients had been excluded from the special case benefit due to unidentifiable diagnosis and disease code with limited patient size.
And from last January, the roster for special case benefit and rare disease medical aid program subject diseases were unified.
The revised regulation also stipulated special case benefit for undiagnosed rare disease patients without a disease code.
Medical aid subject disease roster expanded significantly from 652 to 927 cases, granting financial support to about 2,600 more patients.
Compared to last year, the Rare Disease Medical Aid Program bumped up this year’s budget and allocated about 32 billion won.
The diagnosis support program covers patients with Genetic Testing Support subject disease and undiagnosed condition.
Also from this year, Rare Disease Regional Care Center Network has been expanded to ten centers, consisting of one Central Support Center and other Regional Support Centers.
To sum it up, the Rare Disease Support Scheme mainly focuses on ▲establishing rare disease list and registration system, ▲increasing medical aid to reduce financial burden, and ▲expanding rare disease diagnosis support and Regional Support Centers.
◆Only 5% of diseases have treatment and NHI coverage rate is still low: Despite the government’s effort, some rare disease patients are still struggling to get access to treatment.
Only about five percent of rare diseases have treatment developed.
Diagnosis and treatment developments are far slower than other general medical conditions, because of small limited number of patients and prospective profit estimated low.
And even if better treatments are developed, many of them are unapproved or non-reimbursed, leaving patients hopeless.
Without guaranteed National Health Insurance (NHI) coverage on treatment, patients and their families would suffer not only from physical pain, but also with financial strain.
More than 80 percent of rare diseases are inherited and patient’s family member show similar conditions.
This vicious cycle leads medical expense in one single household to surge exponentially.
However, the current Rare Disease Management Act does not specifically stipulate expansion of NHI coverage on rare disease treatments.
Related industry claims Korea lacks a legal basis to back policy and regulation to boost pharmaceutical accessibility for rare disease patients catering unique qualities of the disease.
Korea may have established a meaningful legislation of Rare Disease Management Act, but in fact, the law does not help many of struggling patients to get better access to drug to this date.
Even at the National Assembly Annual Audit session, lawmakers urged the government to enhance NHI coverage on rare disease.
Lawmaker Yoon Jong-pil of Liberty Korea Party spoke at Ministry of Health and Welfare audit session and pointed out, “Considering exceptionally limited number of patients and difficulty in developing effective treatment, rare disease treatment should be reviewed with more flexible criteria or they would not be accessible to patients in need”.
.Drug pricing department of a multinational pharmaceutical company claimed, “An independent set of reimbursement review criteria should be designed for rare disease treatment as it is impossible to evaluate with common economic sense
.And this is why special clause or supplementary article as a part of Rare Disease Management Act should stipulate expansion of coverage on rare disease treatment”
.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









