- LOGIN
- MemberShip
- 2025-12-24 09:53:52
- Expanded reimb for Keytruda·Dupixent
- by Jung, Heung-Jun | translator | 2025-12-24 08:08:06

As the review of expanded reimbursement for Keytruda (pembrolizumab) and Dupixent (dupilumab) has passed the Health Insurance Policy Review Committee (hereafter referred to as the committee) today (DEC 23), the out-of-pocket cost will drop by up to 95% starting next year.
The committee also finalized a restructuring of the compensation system for clinical laboratory testing CDMOs, the formulation of an adjustment system for relative value units, and the launch of an 'innovative pilot project for community-based primary care', set for the second half of next year.
The Ministry of Health and Welfare (MOHW) approved expanding health insurance coverage for Keytruda and Dupixent during the committee. While Keytruda was previously covered for four cancer types, including non-small cell lung cancer (NSCLC), coverage will now extend to 17 therapies across nine additional cancer types.
Specifically, the expanded coverage includes head and neck cancer, gastric cancer, esophageal cancer, endometrial cancer, small bowel cancer, biliary tract cancer, colorectal cancer, triple-negative breast cancer (TNBC), and cervical cancer.
For patients meeting the eligibility criteria for these expanded indications, the annual out-of-pocket cost per patient is expected to drop significantly, from approximately KRW 73.02 million to KRW 3.65 million (with a 5% co-payment for monotherapy).
Dupixent, which was previously covered for chronic severe atopic dermatitis, will also be covered for severe Type 2 inflammatory asthma starting in January. The annual cost for severe asthma patients is expected to decrease from KRW 15.88 million to approximately KRW 4.76 million (based on a 30% co-payment).
The committee also concluded this year's reimbursement re-evaluation for eight therapeutic ingredients. The reimbursement for spherical adsorptive carbon and artemisia herb extract will be maintained due to price reductions.
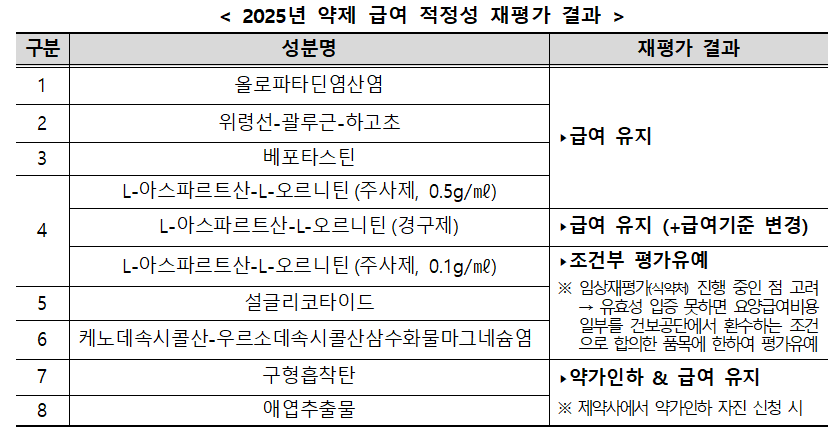
The oral formulation of L-aspartate-L-ornithine will remain covered, though its reimbursement criteria will be restricted to hepatic encephalopathy. For three other ingredients, including sulglicotide, the evaluation has been deferred on the condition that a portion of the reimbursement will be recouped if clinical trials fail to prove efficacy.
Abolition of clinical lab management fees... new institution-specific fees established
The compensation structure for CDMO clinical laboratory testing will be reformed. The consignment fee, which overlaps with existing testing fees, will be abolished, and new fees specific to the CDMO institutions will be established. Furthermore, the billing and payment system will be improved to prevent diagnostic fee discounting.
The CDMO fee criteria will be determined based on ▲the current management fee ▲the respective roles of the institutions ▲the financial impact during the regular RVU adjustment process. The KRW 240 billion saved from the abolition of the management fee will be reallocated to increase reimbursement for under-compensated areas, such as consultation fees.
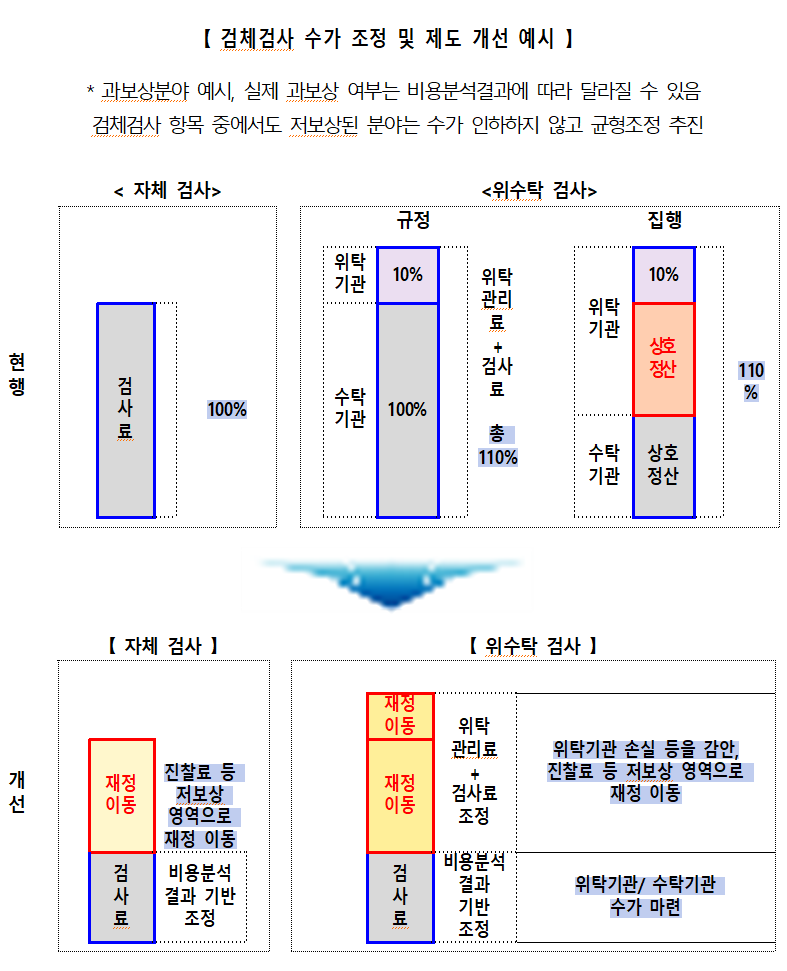
The MOHW plans to revise relevant CDMO regulations in the first half of next year and implement the changes in alignment with the regular relative value unit adjustment cycle. Certification standards for clinical laboratory testing will also be updated.
Regular adjustment of the relative value unit (previously updated every 5-7 years)
To rationalize the fee compensation system, the government will formulate a regular adjustment model for the relative value unit. Previously, relative value unit restructuring occurred every 5 to 7 years, which critics argued failed to reflect rapid changes in medical practice.
The government will review under-compensated and over-compensated services based on medical cost analysis and adjust them to balanced fees.
In particular, funds from adjusting for over-compensation in areas such as clinical laboratory tests and imaging (CT and MRI) will be redistributed to under-compensated basic medical services, such as consultation and hospitalization fees at clinics and hospitals. Funds will also be directed toward strengthening compensation for essential healthcare, including surgeries and care that are severe·emergency and pediatrics·deliveries.
Medical cost analysis results for relative adjustment...Low margins for drug administration and dispensing
The committee discussed the 2023 medical cost analysis results calculated by the Medical Cost Analysis Committee.
These results will serve as the foundation for the 2026 regular RVU adjustments. The '2023 Fiscal Year Cost Analysis Report', which for the first time includes cost-to-revenue ratios by healthcare institution type and specific fee items, is scheduled for publication in the first quarter of next year.
The analysis was expanded to include tertiary hospitals and clinics in addition to general hospitals. The report calculated the cost-to-revenue of reimbursements following the establishment of standardized medical cost calculation guidelines.
Key findings regarding the cost-to-revenue ratio for covered services (based on tertiary hospitals) showed that clinical laboratory fees (192%), special radiologic imaging fees (169%), and radiation therapy fees (274%) had relatively high profit margins compared to costs.
Conversely, drug administration and dispensing fees (11%), basic physical therapy (33%), and basic consultation fees (63%) generated significantly lower revenue than costs.
'Innovative Pilot Project for Community-based Primary Care' to launch in July
The pilot project will launch next year, initially targeting patients aged 50 and older who require integrated management, with plans for gradual expansion.
Under this project, registered patients can receive personalized preventive care, disease and medication management, and lifestyle coaching at their designated clinics, linked to their health check-up results. When necessary, patients can be referred to appropriate medical institutions or receive home-based primary care.
Clinics that complete the required training are eligible to participate. Institutions capable of providing multi-professional, multidisciplinary team support can join as hub institutions.
The government will introduce the 'Primary Care Functional Enhancement Integrated Fee', which compensates for 'patient registration and continuous management efforts' rather than traditional fee-for-service. The MOHW also plans to pilot multidisciplinary team-based service support and performance-based rewards.
The pilot program is scheduled to run for three years, from July 2025 to 2028, with plans to expand its scope to additional regions and institutions starting in 2029.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









