- LOGIN
- MemberShip
- 2026-01-08 18:34:42
- 3 new K-biosimilar approvals last year…second-highest
- by Chon, Seung-Hyun | translator | 2026-01-06 08:25:23
Last year, South Korean biopharmaceutical companies received approval of three new biosimilar products. It was the second-highest annual output following the record-breaking eight approvals in 2024. Samsung Bioepis and Celltrion have seen their follow-up pipelines reach the commercialization stage one after another, while Sam Chun Dang Pharm successfully entered the market with its first biosimilar, expanding the number of domestic biosimilar players to five. Currently, Celltrion and Samsung Bioepis received 11 approved biosimilars each.
According to industry sources on the 5th, domestic firms obtained approval from the Ministry of Food and Drug Safety (MFDS) for four new biosimilar products last year. Samsung Bioepis secured two of these approvals, while Sam Chun Dang Pharm obtained one.
Samsung Bioepis received approval for Obodence (a biosimilar to Prolia) in April 2025, followed by Xbryk (a biosimilar to Xgeva) in May. Both Prolia and Xgeva were initially developed by Amgen, using the same active ingredient, denosumab, but with different dosing schedules and administration cycles. Prolia is used for treating osteoporosis, whereas Xbryk is indicated for preventing skeletal-related events in patients with bone metastases and for treating giant cell tumors of bone.
In September 2025, Sam Chun Dang Pharm's Vgenfli, a biosimilar to Eylea (aflibercept), cleared its final regulatory hurdle. This product is indicated for ophthalmic conditions such as wet age-related macular degeneration, macular edema following retinal vein occlusion, and diabetic macular edema. This marks Sam Chun Dang Pharm's first domestic biosimilar approval, entering a competitive field where Samsung Bioepis and Celltrion had already secured approvals for their respective Eylea biosimilars in 2024.
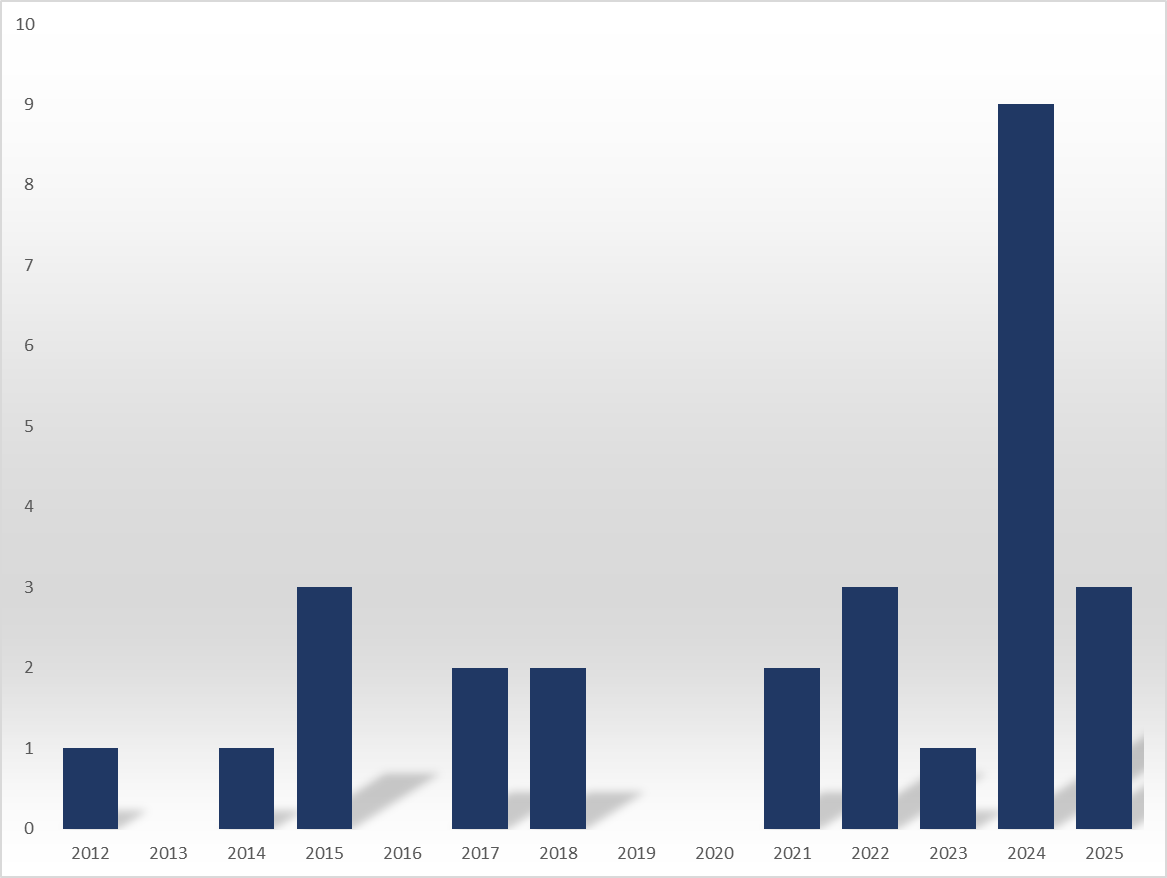
The three biosimilar approvals in 2025 were the second-highest in record. It recorded 9 in 2024, and the previous high was 3 in 2015 and 2022.
Beyond new product entries, domestic firms are continuously strengthening their market competitiveness through supplemental formulation approvals.
Celltrion obtained approval for an Auto-Injector (AI) formulation of Omlyclo in December 2025. This followed the June 2024 approval of the pre-filled syringe (PFS) version. Omlyclo is a biosimilar to Xolair (omalizumab), used for chronic idiopathic urticaria and asthma. The AI formulation is a unique option not currently offered by the original manufacturer in Korea, designed to enhance treatment accessibility for patients who find it challenging to visit medical institutions.
Similarly, Celltrion added two new types of Avtozma (an Actemra biosimilar) in February 2025.
Samsung Bioepis received approval for its Eylea biosimilar, Afilivu, in 2024, and introduced a pre-filled syringe version last year.
To date, South Korean biopharmaceutical companies have successfully commercialized 27 products across 15 therapeutic areas.
Celltrion pioneered this space in 2012 with Remsima and has since secured approvals for biosimilars referencing Herceptin, MabThera, Humira, Avastin, Eylea, Stelara, Xolair, Prolia, Xgeva, and Actemra.
Samsung Bioepis began its journey in 2015 with Etoloce (Enbrel biosimilar) and has successfully expanded into areas referencing Remicade, Humira, Herceptin, Avastin, Lucentis, Soliris, Eylea, Stelara, Prolia, and Xgeva.
LG Chem and Chong Kun Dang have also made notable entries into the Enbrel, Humira, Nesp, and Lucentis markets.
Celltrion and Samsung Bioepis received approval for 11 biosimilars each. Celltrion and Samsung Bioepis account for 85% of the 26 approved products.
Recently, traditional pharmaceutical companies have increasingly joined forces with these biosimilar developers for domestic distribution.
While Samsung Bioepis initially partnered with MSD Korea, it later transferred rights to Yuhan Corp for several autoimmune treatments. However, in March 2024, Samsung Bioepis established its own internal sales organization for direct distribution. It currently maintains partnerships with Boryung for its oncology portfolio (Samfenet and Onbevzi) and Samils Pharmaceutical for its ophthalmology products. For Obodence, Samsung Bioepis selected Hanmi Pharmaceutical as its marketing partner, sharing domestic sales responsibilities.
In 2017, Samsung Bioepis initially selected Daewoong Pharmaceutical as the sales partner for Samfenet but switched the distributor to Boryung in 2021. Immediately following the domestic approval of the Avastin biosimilar Onbevzi in 2021, the company signed an exclusive domestic sales agreement with Boryung. For its ophthalmic disease treatments, Samsung Bioepis chose Samil Pharmaceutical as the sales partner for its Lucentis and Eylea biosimilars.
Samsung Bioepis recently selected Hanmi Pharmaceutical as the sales partner for Obodence. Samsung Bioepis acts as the developer responsible for production and supply, while both companies jointly manage domestic marketing and sales activities.
Daewoong Pharmaceutical entered into a joint sales and distribution agreement with Celltrion Pharm to begin domestic sales of Celltrion's Prolia biosimilar, Stoboclo. Daewoong Pharmaceutical is conducting joint sales of Stoboclo across general hospitals and clinics nationwide alongside Celltrion Pharm. While Celltrion previously sold its biosimilars in the domestic market exclusively through its affiliate, Celltrion Pharm, Stoboclo is the first product from a pharmaceutical company other than Celltrion Pharm to be distributed in the domestic market. Additionally, Daewoong Pharmaceutical has joined LG Chem's sales efforts for its Humira biosimilar, Xelenka.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.








