- LOGIN
- MemberShip
- 2025-12-22 02:41:02
- Policy
- Started a demand survey for drugs participating in the pilot
- by Lee, Tak-Sun Mar 31, 2023 06:08am
- Due to the burden of simultaneous data submission, there are likely to be fewer participating drugs. The government started a demand survey for drugs to participate in the parallel trial project of 'Permission Evaluation Negotiation', which is being pursued rapid reimbursement. The project was planned to drastically shorten the registration period by simultaneously proceeding with benefit evaluation and negotiations from the MFDS approval application stage. According to the industry on the 30th, the Ministry of Health and Welfare is conducting a drug demand survey to participate in a parallel pilot project in 2023, including application for permission, evaluation of benefits, and negotiation of drug prices, as part of a plan to improve access to treatment for expensive severe diseases and strengthen reimbursement management. Candidate drugs are scheduled to apply for approval from the Ministry of Food and Drug Safety in 2023 and are drugs aimed at treating diseases that threaten survival (life expectancy is less than one year) or rare diseases. If there is no existing treatment or if there is a clinically significant improvement in efficacy, etc. compared to existing treatment, participation is possible. The data to be submitted are related to approval: ▲the expected date of application for domestic approval, ▲effectiveness and effect among items of application for domestic approval (scheduled) , ▲countries approved in foreign countries and items permitted, ▲consistency between items approved in foreign countries and items scheduled for application for domestic approval. Regarding disease information of approved indications, ▲ disease severity, life expectancy, survival rate, progress, etc. ▲ existing treatment methods, and treatment performance data for the disease. Regarding clinical usefulness, ▲a clinical trial summary of the therapeutic confirmatory trial (or related evidence source), ▲the expected number of patients, ▲A8 listing status and listing price, ▲benefits evaluation result in excluding countries, ▲cost when applying for decision Effectiveness application track expected data (PE, medication cost comparison, etc.) can be submitted. The data will be used as basic data for selecting drugs for pilot projects. Applications must be submitted to KPBMA by April 4th. The government plans to promote the pilot project in earnest from the second half of the year. On the 28th, Yoo Mi-young, head of the Pharmaceutical Management Office of the HIRA, said at a meeting with the Korea Special Press Association, “We are currently discussing the target selection and related procedures with the relevant departments of the Ministry of Food and Drug Safety to promote the pilot project.” Explained. It is expected that there will be not many drugs to participate in the pilot project. Since the evaluation methods and conditions of the three agencies - MFDS, HIRA, and NHIS - are different, related pharmaceutical companies are also burdened with preparing data at the same time. It is interpreted that the first step was to investigate demand before starting the pilot project in earnest in the second half of the year.
- Policy
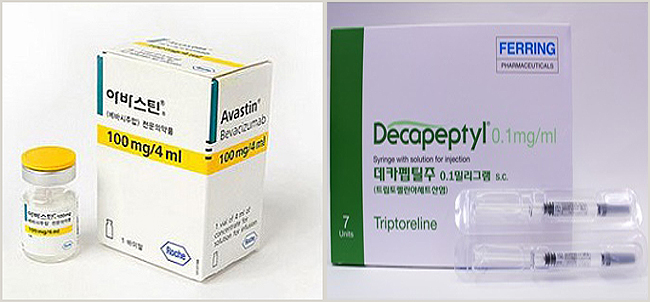
- About 180 clinics without IRB used diagnostic drugs
- by Park,Yang-myeong Mar 30, 2023 05:42am
- Avastin and Decapeptyl, are drugs that allow the use of drugs beyond the scope of approval without the IRB. How much is it used in front-line medical institutions? About 230 macular degeneration treatment drugs Avastin and precocious puberty drug Decapeptyl injection were found to be using these drugs without IRB at 180 hospitals and clinics. The Pharmaceutical Management Office of the Health Insurance Review and Assessment Service disclosed the current status at a meeting with the Special Press Association held on the 28th. In 2019, the relevant notice was changed so that drugs exceeding the permitted scope could be used without reimbursement even without an IRB, but it was only last year that the system was actively applied to the field. The first drug approved by an institution not implemented by the IRB is Avastin, which is used for ophthalmic diseases. Avastin, as a targeted anticancer drug, has been used for various cancers, including colorectal cancer, breast cancer, non-small cell lung cancer, renal cell cancer, ovarian cancer, peritoneal cancer, and cervical cancer. In February 2020, the Korean Academy of Ophthalmology applied for the use of Avastin over the permitted scope to the ophthalmologist, and the HIRA prepared guidelines after discussing infection control measures. The guidelines include measures to prevent infection during the dispensing process. The HIRA received applications for use from non-IRB organizations since February of last year, and until last year, about 230 places used Avastin for ophthalmologic diseases. The second approved drug is Decapeptyl 0.1mg, which is used to diagnose precocious puberty. Decapeptyl is a prescription drug approved for hormone-dependent prostate cancer, endometriosis, uterine fibroids, and central premature onset of puberty in girls under 9 years old and boys under 10 years old. It was not approved as a diagnostic reagent for precocious puberty, but as the supply of the only diagnostic reagent was stopped, medical institutions are continuing to apply for approval for excessive use. It was applied by the Korean Hospital Association in October 2021, and it was confirmed that about 180 hospitals and clinics without an IRB applied for use between July last year and just five months. On the other hand, for the use of drugs over the scope of permission by institutions that have not implemented the IRB, the Korean Medical Association, the Korean Hospital Association, the Korean Dental Association, and related academic societies for each specialty apply for use over permission, and the HIRA reviews for approval. ▲ More than one-third of approved drugs compared to all drug clinical trial institutions for the same case, ▲approval of more than 3,000 drugs for the same case based on the previous year, ▲ Need for expansion Approval can be made if it meets at least one standard, such as medicines for rare diseases and pediatric diseases that are recognized.
- Policy
- New Pompe disease drug Nexviazyme is approved in Korea
- by Lee, Hye-Kyung Mar 30, 2023 05:41am
- The long-term enzyme replacement therapy for Pompe disease, Nexviazyme Inj (avalglucosidase alfa-ngpt), has received marketing authorization in Korea. On the 29th, the Ministry of Food and Drug Safety (Minister Yu-Kyung Oh) announced that it had approved Sanofi-Aventis Korea’s Nexviazyme, a treatment for a rare condition called Pompe disease. Nexviazyme is a recombinant enzyme used as a long-term enzyme replacement therapy in patients confirmed with Pompe disease, which is caused by a genetic deficiency or dysfunction of the lysosomal enzyme acid alpha-glucosidase (GAA). Pompe disease is a rare genetic disorder that causes progressive weakness in the heart and skeletal muscle, leading to respiratory failure and cardiomyopathy. It is a rare condition reported in 1 in 40,000 around the globe and affects about 1,300 people in Korea. The disease presents in two main forms: infantile-onset Pompe disease (IOPD) which presents during infancy, and late-onset Pompe disease (LOPD) which is characterized by onset at all ages and progressively weakens the patients’ muscles. The muscle damage also leads to heart failure, respiratory failure, movement disorders, and sleep disorders. In particular, as Pompe disease is caused by a disorder in the GAA gene, a drug that targets the mannose-6-phosphate (M6P) receptor that allows the transportation of GAA enzymes into the lysosome is administered for its treatment. The drug is a biobetter that has improved dosage and administration over the company’s previous Pompe disease treatment, ‘Myozyme (alglucosidase alfa).’ The company had changed the sugar structure of Myozyme to improve cell absorption of Nexviazyme's active pharmaceutical ingredient. Patients who did not see an effect with Myozyme may use Nexviazyme Inj and see an effect. The MFDS said, “We will continue to make efforts to allow the prompt provision of treatments with confirmed safety and efficacy based on regulatory science."
- Policy
- Obizur will be evaluated for reimbursement upon approval
- by Lee, Tak-Sun Mar 30, 2023 05:41am
- Obizur, a treatment for acquired hemophilia A for adults, approved on the 20th, immediately began a benefit evaluation. This drug is attracting attention as it is the first treatment to replace blood coagulation factor 8 with an indication for acquired hemophilia A. According to the industry on the 29th, the HIRA received an application for Obizur benefits and entered the evaluation. Obizur received approval from the MFDS on the 20th. This drug was designated as an orphan drug in July 2021. US FDA approval was obtained in 2014. Acquired hemophilia A is a rare disease in which autoimmune antibodies cause antibodies to coagulation factor VIII, and bleeding cannot be stopped like in hemophilia patients. The prevalence rate is 0.2 to 1.48 per year per 1 million people, and it is known that there are about 60 patients in Korea. Takeda explains that Obizur is a genetically engineered product made by removing the B-domain from pig coagulation VIII, which is similar to human, and replaces inactivated human VIII, which is not easily recognized by autoimmune antibodies, to help blood coagulation and help control bleeding. did. In phase 2/3 for 28 patients with acquired hemophilia A, Obizur stopped or reduced bleeding in all early bleeding episodes, showed clinical improvement, or showed VIII activity above the target 24 hours after the first administration. The treatment success rate at the time of final administration was 85.7%, and the success rate was higher in the patient group using Obizur as the first treatment at 94%. The patient group using the second-line medicine showed a 73% treatment success rate. It seems that Obizur applied for reimbursement to the HIRA immediately after passing the safety and efficacy verification of the Ministry of Food and Drug Safety.
- Policy
- Govt seeks to improve the drug price reduction system
- by Lee, Tak-Sun Mar 29, 2023 06:02am
- The Korean government will be working together with the pharmaceutical industry to come up with plans to improve the price reduction system that discounts the actual transaction price of drugs. The Health Insurance Review and Assessment Service plans to hold a roundtable meeting with the pharmaceutical industry to improve the actual drug transaction price reduction system based on the recently disclosed research service results. However, whether the improvements will be received with content by both the authorities and the industry remains in question as some of the research service results may be difficult for the pharmaceutical industry to accept. According to industry sources on the 21st, the results of the 'Study to Prepare a Comprehensive Improvement Plan through an Effect Evaluation of the Transaction Drug Price Reduction System' that was led by principal investigator Jin-Hyun Kim, a Seoul National University professor, was disclosed through the public institution management disclosure system on the 9th. The research team proposed short-term, mid-term, and long-term measures for improvement in its final report. As for the short-term measures, ▲abolishing the 10% upper limit in the price ceiling system and ▲reflecting the low purchasing price paid by national and public hospitals in the drug price cuts were proposed. As mid-term measures, ▲ identifying the actual transaction price including rebates ▲ tougher punishments for false reporting, ▲ real financial savings, and ▲ promoting low-price purchases, etc. were proposed. As long-term measures, ▲ switching to a list price reimbursement system rather than the currently ineffective actual transaction price reimbursement system, ▲reducing the insurance price ceiling based on the actual transaction price, ▲changing the drug price structure including the dispensing fee, and ▲ maximizing pharmaceutical expense saving effect on outpatient pharmaceutical expenditures were proposed. As the proposed mid-to long-term measures are difficult to apply immediately, the short-term plans proposed by the research team are highly likely to be discussed with the pharmaceutical industry as a promotion task. However, predictions are that it will be difficult to reflect the low purchasing price paid by national and public hospitals in the drug price cuts due to strong opposition from the industry. Therefore, realistic measures such as the abolition of the 10% upper limit in the price ceiling system are expected to be discussed. A HIRA official said, “The actual drug transaction price reduction system has been in effect for a long time, and there had been criticism on its low effect, therefore, we plan to come up with an improvement plan. If discussions progress well, measures may even be derived within the year.” The reductions to the actual drug transaction price had been carried out every year in line with the separation of prescribing and dispensing system in 2000 to reflect the actual transaction price of drugs in the drug price, and then has been conducted every two years since 2016. However, the prevailing opinion was that an improvement was needed as its drug cost reduction effect was poor and it was difficult to identify the actual transaction price. This was why HIRA set out to derive an improvement plan through a research service last year.
- Policy
- 87.5% of anticancer/rare drugs, evaluated as drugs w/o PE
- by Lee, Tak-Sun Mar 29, 2023 06:01am
- Yoo Mi-yeong, head of the HIRAAs the PE drug standard expands, the HIRA is seeking a reasonable management plan. This year, we plan to improve and supplement the PE system by deriving detailed plans through research services. Yoo Mi-young, head of the Pharmaceutical Management Office at the Health Insurance Review and Assessment Service, explained this at a meeting with the Professional Reporters Association held on the 28th. "In the case of anti-cancer drugs and rare disease drugs that were listed as new drugs last year, 87.5% of the total were evaluated as drugs without PE," said Director Yoo. We plan to prepare management plans based on this.” The research service is planned to start in April and end around the end of the year, and it is a policy to come up with a reasonable plan to improve health insurance financial sustainability and patient accessibility. The system that allows PE data submission to be omitted began in May 2015 with drugs for treating rare diseases for which there is no alternative or that threatens survival, and anticancer drugs, and in October 2020, it was expanded to drugs for tuberculosis, antibacterial drugs, and emergency antidotes among essential national medicines. From January of this year, it has been applied to drugs that have been proven to improve the quality of life of pediatric patients with rare diseases. Accordingly, CRYSViTA, a pediatric rickets treatment, falls under this category, passed the HIRA review stage without PE, and is currently negotiating with the NHIS. This PE omission system is being used to advance the drug evaluation period in order to increase patient access to treatment. Starting in the second half of this year, full-scale pilot projects linking permission-evaluation-negotiation For the same purpose, the MFDS, the HIRA, and the NHIS are starting this year to shorten the registration period for treatments for severe diseases such as cancer and rare diseases. are promoting Director Yoo explained, "We are currently in discussions with the MFDS related departments on target selection and related procedures to promote the pilot project." However, since the three institutions have different evaluation methods and conditions, and the beneficiary pharmaceutical companies also have burdens in preparing data at the same time, it is expected that only a few pharmaceutical companies will actually benefit from the system. Participation in the NHIS Pharmaceutical Evaluation Committee, there are concerns that fairness and objectivity issues may be raised. On the 7th, Lee Sang-il, executive director of the NHIS, said at a Korea Special Press Association meeting, “Secure the benefit adequacy and consistency of financial impact when listing a new drug, and evaluate the uncertainty of risk-sharing new drugs through organic linkage of negotiations. In order to support the decision, participation in the Pharmaceutical Reimbursement Evaluation Committee of the NHIS is necessary, so we plan to review a plan for NHIS members to participate in the Pharmaceutical Reimbursement Evaluation Committee through consultation with the Ministry of Health and Welfare and related organizations such as the HIRA." Director Yoo said, "Currently, the NHIS attends and monitors every meeting, and regularly holds meetings with the Ministry of Health and Welfare and the NHIS to discuss the contents of the committee's deliberations and issues for each individual agenda, and shares related data from time to time." Negative views were made on the need for NHIS to participate in the committee.
- Policy
- Erleada is listed
- by Lee, Tak-Sun Mar 28, 2023 05:56am
- Janssen Erleada, a new drug for the treatment of prostate cancerJanssen Korea, which succeeded in listing Erleada, a new prostate cancer treatment drug, voluntarily lowers the upper limit on Zytiga, an existing prostate cancer treatment drug. The industry believes that another so-called 'trade-off' case appeared last year when MSD lowered the upper limit on its drug while expanding reimbursement for the immuno-oncology drug 'Keytruda'. According to the industry on the 22nd, Janssen Erleada will be listed next month at a marked price of 20,045 won as a risk-sharing agreement (RSA) reimbursement type. In terms of the market price alone, it is slightly cheaper than the competing drug, Xtandi, which costs 20,882 won. However, it is evaluated that Erleada is economical for patients in that Xtandi is a selective benefit with a co-payment of 30%, while Erleada is a mandatory benefit with a co-payment of 5%. Some predicted that Erleada would not be easy to apply for benefits while receiving PE, unlike Xtandi. In fact, Erleada and Xtandi passed the HIRA in February of last year, but Xtandi, which omitted PE and accepted selective benefits, applied for benefits in August of that year, but Erleada had to pass the year. As a result, Erleada's benefit is also evaluated as a strategic success on the part of Janssen. It is interpreted as suggesting a trade-off as one of the strategies. It appears that the company negotiated with the insurance authorities by lowering the upper limit on Zytiga, an existing prostate cancer treatment, as a condition for Erleada's insurance coverage. Zytiga will cut 4.7% from next month from 17,606 won to 16,780 won. An industry insider said, “Trade-off negotiations in the form of drug price cuts for existing treatments through new drug reimbursement are gradually expanding following Keytruda last year.” It can be seen as a kind of trade-off negotiation in that it is intertwined with
- Policy
- Jeil Pharmaceutical discontinued Actos co-promotion
- by Lee, Tak-Sun Mar 27, 2023 05:56am
- Actozon 15mgJeil, which has already launched sales of the generic drug Actozone 15mg and the combination drug Actozonemet 15/850mg from 4Q last year, is expected to launch Actozon 30mg in the reimbursement market in April. According to the industry on the 26th, Actozon 30mg will be listed at the upper limit of 940 won from April 1st. There are still fewer than 19 generic drugs listed, and Jeil Pharmaceutical succeeded in calculating the same price as the highest price by meeting all the standard requirements. Previously, when Actozon 15mg was re-listed in January of last year, it was difficult to calculate the highest price as there were more than 20 generics. Accordingly, Actozon 15mg has been listed at the lowest price of 359 won for the same product. Actozonemet was also listed at the highest price of 714 won when it was re-listed in August of last year, as fewer than 19 identical drugs were listed. In terms of drug price, apart from 15mg, single drugs 30mg, and combination drugs maintain the highest price, and it is evaluated that they did well even though they entered the generic market belatedly. Jeil entered the generic market belatedly because the co-promotion of the original Actos and Actos met, which had been sold since the second half of last year, was discontinued. Best has sold Actos since 2013. At the time, it signed a co-promotional contract with Takeda. Jeil had to end 9 years of original sales as Celltrion Pharm took over the Asia-Taeyang region rights for Takeda products including Actos last year and started selling them alone from the second half of the year. Jeil immediately put generics into the market. In January of last year, Actozon 15mg, which had been removed from reimbursement, was listed again on the list of benefits, and in August of that year, Actozonemet was also listed again. Then, from the fourth quarter of last year, generic drugs were sold in earnest. Actozon 30mg was additionally approved in January. From April, it is expected to enter the market in earnest with a full lineup of pioglitazone capacities. As Jeil secured a large number of customers by selling the original Actos, it is expected that the relationship with customers lost due to the suspension of product sales in the generic business will be restored in the short term. The three-drug regimen including Metformin + SGLT2 will be covered from April for Glitazone drugs, which is also positive for sales recovery. An official in the pharmaceutical industry said, “As Jeil has a long experience in selling Actos, it is highly likely that it will quickly settle into the market as a generic drug.” He explained, "Therefore, other generic companies are also watching Jeil's movements."
- Policy
- Dupixent 200mg, newly listed
- by Kim, Jung-Ju Mar 27, 2023 05:55am
- Sanofi-Aventis Korea's severe atopic dermatitis treatment Dupixent PFS 300mg has expanded insurance coverage to pediatric and adolescent patients, and products with 200mg content will be newly listed next month with an expanded range. Taking this as an opportunity, the government is planning to expand the scope of benefits for special calculations based on eliminating blind spots in existing treatments for severe atopic dermatitis. Janssen Korea Erleada, which is used for the treatment of metastatic prostate cancer (mHSPC), will be newly listed as a risk-sharing contract (RSA) reimbursement type at around 20,000 won. According to the industry on the 21st, the Ministry of Health and Welfare plans to revise the 'drug reimbursement list and reimbursement upper limit table' and is pushing to apply it on the 1st of next month. There are a total of 4 new drugs being promoted, 1 drug that has been expanded in the scope of use (benefit), and 4 items that are under contract renewal negotiations with the NHIS due to the expiration of the RSA period. ◆Newly listed item = Erleada is newly listed next month at 20,045 won so that it can be applied for health insurance. This drug is used in combination with androgen deprivation therapy (ADT) for the treatment of patients with hormone-responsive metastatic prostate cancer. Currently, A7 is registered in a total of seven countries, including the United States, Japan, France, Germany, Italy, Switzerland, and the United Kingdom. The company applied for insurance listing to the Review and Assessment Service on November 30, 2021, a year later. proceeded. At the time, the committee judged that the cost-effectiveness ratio was included in the acceptable range as a result of the economic evaluation, but the reimbursement was appropriate when the drug price was lowered in consideration of the financial impact such as the possibility of an increase in market share. As the company accepted this result, the drug was put on the negotiating table of the NHIS, and immediately entered into negotiations on the drug price and expected billing amount, and was successfully registered in April. The corporation expects that this drug will be cheaper than alternative treatments, and actual additional finance will be insignificant when considering the RSA refund rate in the future. Dupixent, which is used for severe atopic skin disease in adults, is also listed as a 200mg product with reduced content. Similar to the 300mg content product already registered and covered by insurance, RSA refund type, total amount limit type, and initial treatment cost refund type were applied to this content product in combination. In addition, Bukwang Pharmaceutical's Zaledeep 5mg and 10mg content products, which have stepped on the track of skipping drug price negotiations with the NHIS, are also listed at 102 won and 153 won per capsule, respectively. ◆ Expansion of scope of use = The scope of reimbursement for Dupixent 200mg, which is already registered, will be expanded from adults currently applied to children and adolescents in the future. Currently, it is estimated that 5,000 Korean adults with atopic dermatitis are receiving benefits. The government plans to broaden the reimbursement standard so that the drug can be widely used for children (6-11 years old) and adolescents (12-17 years old) with atopic dermatitis. The number of these patients is estimated to be 2550. The drug's benefit for children and adolescents is being applied to all A7 countries, and it is also being applied to adults in Korea, so its clinical usefulness has already been proven. Following the increase in benefits this time, the company negotiated with the NHIS again and set the price at 696,852 won per share, a 1.5% cut. At the same time, the government expands the scope of application of special calculations accordingly. It is to adjust the benefit standard while expanding the benefit from the existing adult target to children and adolescents. This is because if the current registration standards for calculation special cases are maintained, pediatric patients fall under the pharmaceutical reimbursement standards, but cannot meet the registration standards for calculation special cases, creating a blind spot where special cases are not applied. ◆ RSA Expiration Evaluation Items = Negotiations were made to renew contracts for 4 items of drugs covered by insurance through RSA. The target is Cabometyx, a renal cell cancer drug from Ipsen Korea, and Erbitux Inj. 5mg/mL (cetuximab), colorectal cancer, and head and neck cancer treatment from Merck. Before the contract period of the RSA drug expires, the government has the Pharmaceutical Review Committee evaluate the drug's clinical usefulness and cost-effectiveness, and proceed with contract renewal negotiations with the NHIS. The types of negotiations include lower drug prices, expected billing amounts, reimbursement rates, and caps. The target drugs for this time were contracts with Cabometyx or Erbitux, which limit the amount of use per patient, and as the companies expressed their intention to renew the contract, the procedure was followed. As a result of the negotiations, Cabometyx will be cut by 2.5% for each content, and Erbitux will be cut by 8.5%. In the case of Erbitux, the new drug price will be applied as of May 1 due to the renewal of the contract.
- Policy
- Forxiga patent is about to expire
- by Kim, Jung-Ju Mar 24, 2023 05:48am
- As the patent for Dapagliflozin, the original drug (Forxiga), is about to expire, domestic generics of this drug will be released early next month. Accordingly, the government is swiftly proceeding with the process of calculating the drug price and additional negotiations so that the drug can be listed immediately. According to the industry on the 21st, the Ministry of Health and Welfare is planning a revision of the drug reimbursement list and the maximum reimbursement amount table and is pushing to apply it on the 1st of next month. The application date is April 8, the day after the patent expiry date of Forxiga. Forxiga is an antidiabetic drug, and its price dropped to 734 won, or 3.4%, after negotiations with the NHIS for PVA type "Na" was concluded on the 13th. Domestic generics, which are taking a breather to secure market share, are 89 single drugs and 78 combination drugs. The Ministry of Health and Welfare has calculated generic drug prices based on 734 won, which was set as a result of PVA negotiations, in order to quickly register these drugs. If the original drug expires, the generics are automatically given a price calculation of 53.55%. Currently, companies ahead of the launch of Forxiga's generics are currently in the process of negotiating with the NHIS for stable supply and quality control while preparing for enlistment on the 8th. The deadline for negotiations is the 30th of this month. Governments and authorities expect generics to quickly capture half of the claims once they hit the reimbursement market. The estimated size of fiscal savings is expected to be around 1.1 billion won per month. Estimated on an annual basis, it is about 13.2 billion won. As the share of domestic products increases, the reduction in health insurance finances is proportional to that amount, so the government has no choice but to be busy.