- LOGIN
- MemberShip
- 2025-12-17 18:44:39
- Policy
- DREC members’ term extended due to delayed nominations
- by Lee, Tak-Sun Sep 10, 2025 06:13am
- The term of office for members of the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service (HIRA), which is responsible for the final drug reimbursement adequacy evaluations, has been extended. Although their term was originally scheduled to end on the 7th, the extension was explained as due to delays in the candidate recommendation process for the next committee members. According to HIRA, the terms of both the 9th DREC and its subcommittee members have been extended. The 9th committee began its term on September 8, 2023, and was set to end on September 7, 2025. Each term lasts two years, and the current committee has 75 members in total. Accordingly, HIRA has been accepting nominations for candidates for the 10th-term DREC from various organizations since last month. DREC members are appointed based on recommendations from academic societies and organizations, with medical and pharmaceutical-related professional societies recommending around 70 members. The Korean Pharmaceutical Association can recommend one expert. However, due to delays in candidate recommendations, the composition of the 10th DREC could not be completed before the expiration of the 9th DREC's term. Consequently, the terms of the 9th DREC members have been extended. DREC’s operating regulations also stipulate that members whose terms have expired shall continue to perform their duties until their successors are appointed. HIRA explained that it plans to promptly finalize the recommendation process and complete the composition of the 10th committee. It maintains that there will be no changes to the DREC's schedule. The next DREC meeting is scheduled for October 2nd, which is likely to be attended by the existing 9th committee members. Once the 10th committee is formed, its subcommittees—including those for reimbursement standards, pharmacoeconomic evaluation, risk-sharing agreement, budget impact assessment, herbal medicines, and post-listing evaluation—will also be reconstituted. The DREC generally consists of around 105 members. The 31 medical societies eligible to recommend candidates include the Korean Society of Cardiology, Korean Society of Gastroenterology, Korean Academy of Tuberculosis and Respiratory Diseases, Korean Endocrine Society, Korean Pediatric Society, Korean Neurological Association, Korean Neuropsychiatric Association, Korean Surgical Society, Korean Cancer Association, Korean Academy of Family Medicine, Korean Dermatological Association, Korean Urological Association, Korean Ophthalmological Society, Korean Society of Otorhinolaryngology – Head and Neck Surgery, Korean Association for the Study of the Liver, Korean Diabetes Association, Korean Society of Nuclear Medicine, Korean Society of Infectious Diseases, Korean College of Rheumatology, Korean Society for Transplantation, Korean Society of Hematology, Korean Association for Lung Cancer, Korean Breast Cancer Society, Korean Gastric Cancer Association, Korean Society of Gynecologic Oncology, Korean Society of Pediatric Hematology-Oncology, Korean Urological Oncology Society, Korean Orthopedic Association, Korean Society of Coloproctology, Korean Society of Medical Oncology, and Korean Society of Surgical Oncology. At each monthly meeting, 20 committee members are randomly selected to participate. In July, HIRA revised its regulations, granting the HIRA President the authority to appoint the committee chairperson and to determine the composition of subcommittees and appoint subcommittee chairs. HIRA explained this as a measure to enhance the effectiveness of the DREC's composition and operation. However, some in the industry have expressed concerns that this could undermine the committee's fairness and neutrality.
- Policy
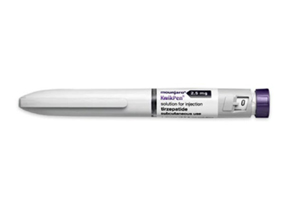
- Six strengths of obesity drug Mounjaro Quick Pen approved
- by Lee, Hye-Kyung Sep 08, 2025 06:16am
- Following its launch in Korea, Eli Lilly has expanded the product lineup of its obesity treatment Mounjaro (tirzepatide) by receiving approval for the Mounjaro QuickPen formulation. On September 5, the Ministry of Food and Drug Safety approved 6 QuickPen doses: 2.5 mg/0.6 ml, 5 mg/0.6 ml, 7.5 mg/0.6 ml, 10 mg/0.6 ml, 12.5 mg/0.6 ml, and 15 mg/0.6 ml. Mounjaro is a once-weekly injectable single molecule that selectively binds to and activates both the GIP and GLP-1 receptors. Treatment is initiated at 2.5 mg (step 1) and increased in increments after at least four weeks. Unlike Saxenda and Wegovy, which are only available as single-pen formulations, Mounjaro is offered in multiple formulations — beginning with pre-filled syringes, now QuickPens, and vials in the pipeline. In the case of the pre-filled formulation, one pen contains one dose, requiring four pens per month. In the case of the Quickpen that was approved this time, one pen now contains four doses, covering an entire month, similar to Saxenda and Wegovy. The vial formulation, which is pending approval, is administered via syringe and needle. While this is less convenient, it is expected to be more affordable. Meanwhile, Mounjaro has faced nationwide shortages within just three weeks of launch, particularly for the starter 2.5 mg dose. Lilly Korea is also pursuing reimbursement for its diabetes indication. In Korea, Mounjaro is approved for glood glucose control in adults with type 2 diabetes (monotherapy or combination therapy); chronic weight management in adults with obesity or overweight with at least one weight-related comorbidity; and adjunctive therapy to diet and exercise for obesity with moderate-to-severe obstructive sleep apnea in adults.
- Policy
- Savings of KRW 32.6B expected from 2025 'Type-Da' PVA nego
- by Lee, Tak-Sun Sep 05, 2025 06:20am
- The National Health Insurance expenditure is expected to see a savings effect of approximately KRW 32.6 billion due to this year's negotiation for the 'Type-Da' Price-Volume Agreement. This year's negotiated items total 110, comprising 48 active ingredients. The ceiling price of these items has been adjusted as of September 1. Items subjected to one-time reimbursement instead of drug price reduction are also included in this list. According to the National Health Insurance Service (NHIS), on September 4, National Health Insurance expenditure is anticipated to be reduced due to the adjusted ceiling price (including one-time reimbursement cases) resulting from the successful negotiation of the 'Type-Da' Price-Volume Agreement. Items included in 'Type-Da' list are those that do not meet criteria for 'Type-Ga' or 'Type-Na.' When a drug has been listed for four years or more, its ceiling price is adjusted through negotiations with the National Health Insurance Service if the claim amount for the same product group increases by more than 60% compared to the previous claim amount, or by more than 10% and exceeds KRW 5 billion. This year, a total of 110 items comprising 48 ingredients were subject to negotiations. This includes several blockbuster products such as Hanmi Pharmaceutical's Rosuzet Tab, Yuhan's Rosuvamibe Tab, Celltrion's Remsima Inj, Organon's Atozet Tab, and Janssen's Concerta OROS Tab. Analysis suggests that the National Health Insurance finances are expected to see savings of approximately KRW 32.6 billion from this year's PVA negotiation. This figure decreased from last year's savings of KRW 52.1 billion, as last year's Type D negotiations included 207 items comprising 63 product types, which was more than this year. Meanwhile, eight drugs, including Daewon Pharmaceutical's Codaewon S Syrup, were signed up for one-time reimbursement instead of a price cut due to temporary usage increases resulting from unavoidable circumstances, such as the COVID-19 pandemic. The one-time refund contract was introduced to promote the stable supply of medicines. Additionally, 12 items from innovative pharmaceutical companies were selected as negotiation targets three or more times within a five-year period, resulting in a 30% reduction in the price cut rate. The NHIS has been implementing this reduction plan since last year to enhance the sustainability of the pharmaceutical ecosystem.
- Policy
- Pilot Drug Approval·Review Coordination Committee extended
- by Lee, Hye-Kyung Sep 04, 2025 06:10am
- The pilot operation period for the Pharmaceutical Approval and Review Coordination Committee will be extended by one year. According to the pharmaceutical industry on September 3, the Ministry of Food and Drug Safety (MFDS) decided to extend the pilot period for the Coordination Committee by one year, starting from August 29. The committee had been operating on a trial basis for one year since June 17 of the previous year. This extension is based on a revision of the committee's operational guidelines. For the past year, applications for adjustment were limited to cases involving safety and efficacy review data, quality review data, and documentation related to data protection. However, starting this year, the scope has been expanded to include 'all matters' for which the Pharmaceutical Approval Management Division has requested supplementary data. The scope of applications for adjustment following the Pharmaceutical Approval and Review Coordination Committee extension. The Coordination Committee was established last June to make the drug product approval and review process more transparent and rational. It allows applicants to request an adjustment when supplementary data is asked for during the drug product approval and review process. The committee, chaired by the Director of the Drug Safety Bureau, includes the Head of the Pharmaceutical Review Department, the Director of the Pharmaceutical Policy Division, the Director of the Pharmaceutical Approval Management Division, relevant review department heads, and experts from the Central Pharmaceutical Affairs Council. They proceed with adjustments through a majority vote. With the pilot program extended for another year, it will apply to applications submitted to the Pharmaceutical Approval Management Division. The MFDS plans to evaluate the operational results at the end of this period to decide whether to terminate the pilot program, transition it to a full-scale operation, or further expand the scope of adjustment applications. The scope of applications for adjustment includes ▲Review data specified in Article 5 of the 'Regulations on Drug Product Approval, Notification, and Review' ▲Data related to the eligibility for clinical trial data protection, as per Article 31-6 of the 'Pharmaceutical Affairs Act' and Article 21-2 of the 'Rules on the Safety of Pharmaceuticals, etc.' ▲Other matters for which adjustment is deemed necessary in response to a request for supplementary information (excluding requests for supplementary data needed for evaluating the implementation status of Good Manufacturing Practice (GMP) and clinical trials). An applicant wishing to request an adjustment must submit an application form, as specified in Annex No. 1 of the guidelines, to the Pharmaceutical Approval Management Division within 30 days of the request for supplementary information. The committee plans to process the adjustment applications, in principle, within the 60-day supplementary request period. Only the applicant listed on the application form can request an adjustment; an agent cannot make this request. Meanwhile, once an application is selected for adjustment, it will be thoroughly discussed at a Coordination Committee meeting, which is composed of both internal and external experts. A two-thirds majority vote of the committee members present decides on the supplementary requirements. The results of the adjustment are then communicated to the relevant divisions and the applicant.
- Policy
- MFDS allocates KRW 812.2 billion for next year's budget plan
- by Lee, Hye-Kyung Sep 04, 2025 06:09am
- The Ministry of Food and Drug Safety (MFDS; Minister, Yu-Kyoung Oh) has announced that its budget for 2026 has been set at a total of KRW 812.2 billion, an increase of KRW 63.3 billion (8.4%) from this year's budget of KRW 748.9 billion. This budget plan was focused on facilitating the smooth execution of the new government's national tasks and restructuring expenditure to ensure efficient financial management. The 2026 MFDS budget plan includes four areas: enhancing safety and expanding the foundation for innovative growth in the pharmaceutical and bio-health sectors, strengthening customized safety support for food and medicine, considering the regulatory environment, creating a safe food and healthy dietary environment, and building a proactive food and drug safety management system for the future. A total of KRW 170.4 billion has been allocated to enhance safety and expand the foundation for innovative growth in the pharmaceutical and biotech sectors. The Fund for the Korea Orphan & Essential Drug Center will increase from KRW 4.5 billion this year to KRW 6.7 billion next year. To resolve the unstable supply of rare and essential medicines, MFDS will strengthen the stable supply foundation by expanding contract manufacturing of discontinued products and the emergency import of self-administered medicines with minimal demand. For supporting and building a management system, such as innovative medical devices, KRW 2 billion will be invested. A newly allocated budget of KRW 15 billion will be invested next year to fund the rapid commercialization of AI-driven products. MFDS will assist in the swift commercialization of promising AI-based products in the food and medical device sectors, thereby shortening Korean companies' development timelines and accelerating their market entry. The budget for the Korean Association Against Drug Abuse has been increased from KRW 16.5 billion this year to KRW 17.1 billion next year. A total of KRW 105.4 billion has been allocated for strengthening customized safety support for food and medicine, taking into account the regulatory environment. With the biohealth industry continuing to grow and the demand for systematic regulatory support from industries with limited experience and expertise increasing, MFDS plans to expand regulatory support by building an integrated consultation platform and securing customized consultation personnel for advanced and next-generation biopharmaceuticals. The budget for this will be significantly increased from KRW 500 million this year to KRW 11.4 billion next year. To lead the development of the pharmaceutical industry, MFDS will create review guidelines for new technologies and concepts, such as AI-driven products, and establish review standards that consider the characteristics of advanced and next-generation biopharmaceuticals. This will secure approval and review capabilities at the level of developed countries. To counter non-tariff barriers, such as country-specific approval regulations that hinder the export of Korean pharmaceuticals, MFDS will operate export approval support hubs. These hubs will analyze and provide case studies of approvals by product and offer regulatory consultations for export countries, thereby supporting the swift acquisition of overseas drug approvals. A new budget of KRW 5.5 billion has been allocated for operating a regulatory science talent development program in which universities, industries, and research institutes participate. The goal is to cultivate regulatory science professionals who can scientifically evaluate the safety of advanced bio-health products. A total of KRW 187.1 billion has been allocated for creating a safe food and healthy dietary environment, and a total of KRW 146.9 billion has been allocated for building a food and drug safety management system for the future. Next year, MFDS plans to build an automated drug approval and review system. This system will verify submitted document requirements, handle repetitive and routine tasks, and generate data summaries and reports. The system is intended to address the shortage of review personnel and expand patients' treatment opportunities through a faster drug approval process. The automation system will be expanded from generic drugs next year to active pharmaceutical ingredients (APIs) in 2027 and new drugs in 2028. To respond to the changing environment, such as online food distribution and the development of artificial intelligence, MFDS will establish an Information Strategy Plan (ISP) to build an "Integrated Food Safety Information Network." This network will consolidate 15 food-related information systems and automate public service and administrative tasks. In addition, as the scope of narcotics investigations expands, MFDS will secure digital forensics personnel and equipment dedicated to investigating medicinal narcotics. It will also broaden the synthesis of standard materials for new psychoactive substances and the evaluation of dependence on temporary narcotics. MFDS stated, "Once the 2026 budget plan is finalized through the National Assembly's deliberation process, we will do our best to execute the new government's national tasks and key projects without delay and protect the health and safety of the public."
- Policy
- HIRA "Reimb application of Wegovy has not been filed"
- by Lee, Jeong-Hwan Sep 04, 2025 06:08am
- Product photo of Wegovy The Health Insurance Review & Assessment Service (HIRA) announced that it will conduct a fair and swift evaluation if an application for reimbursement is submitted for Novo Nordisk's popular obesity drug, Wegovy (semaglutide). HIRA clarified that since Wegovy's company has not yet applied for reimbursement, HIRA is yet at the stage of determining Wegovy's National Health Insurance reimbursement. HIRA stated recently after a recent inquiry from Rep. Kim Seon-min of the Cho Kuk Innovation Party regarding the reimbursement status of Wegovy. Rep. Kim had asked HIRA for its stance on a plan to transition the non-reimbursed prescription drug Wegovy to National Health Insurance coverage and management. Rep. Kim likely inquired about the management plan related to the significant prescription volume of Wegovy, which has garnered immense popularity since its launch in Korea. According to data from the Drug Utilization Review (DUR) system, approximately 400,000 prescriptions for Wegovy have been issued in the roughly eight months since its launch in October 2024, which translates to about 80,000 prescriptions per month. The non-reimbursed prescription price for Wegovy ranges from KRW 200,000 to 300,000 for the 0.25mg, 0.5mg, and 1.00mg doses, and over KRW 400,000 for the 1.70mg and 2.40mg doses. Related to this, Rep. Kim has raised the need for managing the side effects that arise from people who are not overweight or obese using these drugs for cosmetic purposes. The medical community has also pointed out that some doctors are prescribing Wegovy and other obesity drugs for cosmetic, rather than therapeutic, purposes. This practice, they argue, can lead to repeated prescriptions and illegal trading of excessively prescribed medicines on online platforms. To address these issues, the medical community is proposing that obesity treatments be brought under the National Health Insurance system, subjecting them to a public monitoring and management system. In response to these concerns, Rep. Kim asked HIRA for its plan to manage the side effects of Wegovy through reimbursement. However, HIRA's response was general and procedural. HIRA explained, "For a new drug to be listed for reimbursement, the pharmaceutical company must first apply for reimbursement to the Minister of Health and Welfare and the head of HIRA, along with the necessary documentation," and added, "Then, this is followed by HIRA will evaluate the drug's clinical utility and cost-effectiveness, followed by drug price negotiations with the National Health Insurance Service. Finally, a notification is issued by the Ministry of Health and Welfare." HIRA said, "Wegovy's manufacturer had not yet submitted a reimbursement application after its approval by the Ministry of Food and Drug Safety," and added, "If an application for this drug is submitted in the future, we will ensure that it is evaluated fairly and swiftly." Meanwhile, the decision on National Health Insurance reimbursement for new obesity drugs, such as Wegovy, will be based on South Korea's health insurance finances, reimbursement equity, and the cost-effectiveness of these drugs.
- Policy
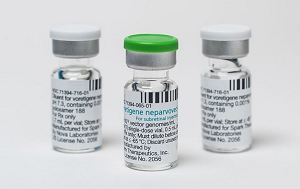
- Luxturna shows signif improvement in 3 out of 6 patients
- by Lee, Tak-Sun Sep 03, 2025 06:09am
- Luxturna (voretigene neparvovec, Novartis), a one-shot gene therapy that costs approximately KRW 300 million, showed clinically significant changes set by reimbursement criteria in only half of the patients. As indicated in the performance evaluation results disclosed last year, the effectiveness was only 50%. The Health Insurance Review & Assessment Service (HIRA) disclosed the latest performance evaluation results for Luxturna on August 29. Luxturna is a gene therapy for patients with inherited retinal disease, administered as a single subretinal injection in each eye. The ceiling price for one vial is KRW 325.8 million, with a patient co-payment of approximately KRW 10.5 million per person. Due to its high cost, health authorities have a risk-sharing agreement (RSA) with three types of contracts (refund, expenditure cap, and performance-based refund) to manage the drug expenditure. The performance-based refund contract, in particular, requires a post-administration performance evaluation to adjust the refund rate. The detailed reimbursement criteria for this drug are as follows. 1. A clinical evaluation (light sensitivity, vision, visual field, etc.) must be performed before administration (within 90 days before the first eye's injection) and at 1-3 months, 12 months, and annually for up to 4 years after administration (after the second eye's injection if both eyes are treated). Objective records, such as medical charts, must be submitted. 2. Light sensitivity must be evaluated using a full-field light threshold test with white light. 3. A clinically significant change is defined as an improvement of 1 log unit (average value for both eyes) or more from the baseline in the full-field light threshold test. The first performance evaluation result was disclosed on October 31 of last year, following the reimbursement listing in February of that year. The first evaluation followed up on four patients 1-3 months after administration. The result showed that two patients had a significant improvement, while two did not, indicating a 50% success rate. The latest evaluation results are based on a total of six patient cases. The evaluation was conducted 1-3 months after administration for two patients and 12 months after for four patients. The results showed that one patient at 1-3 months and two patients at 12 months had a significant improvement. In contrast, it was evaluated that one patient at 1-3 months and two patients at 12 months did not meet the criteria for significant improvement. It means that half of the patients were successful, while the other half failed. Since Luxturna's performance evaluation will continue for up to 4 years, the efficacy is expected to be more accurately verified as more data accumulates. By adjusting the refund rate accordingly, the high cost of the drug can be controlled. HIRA is currently conducting performance evaluations for other high-cost drugs, including Kymriah, Zolgensma, and Qarziba.
- Policy
- Bill to mandate generic substitutions gains momentum
- by Lee, Jeong-Hwan Sep 03, 2025 06:09am
- On September 2, the ruling party introduced a bill to mandate generic (ingredient-based) prescriptions for medicines with unstable supply. The bill establishes the legal basis for “shortage drugs” and allows prescribing by ingredient name instead of brand name. The bill holds significance in that it amends the Pharmaceutical Affairs Act to define drugs frequently in shortage and sets out legal procedures to designate such through public-private consultations, and amends the Medical Service Act to legislate the legitimacy and standards for mandatory ingredient prescriptions. In other words, the bill enforces a law that stipulates that “drugs in such unstable supply that generic prescribing must be enforced” through deliberations by a public-private consultative body. Representative Jong-tae Jang of the Democratic Party of Korea, who spearheaded the amendments, has stated he will push for their swift passage. The participation of fellow Democratic Party representative Yoon Kim (a physician) and Rebuilding Korea Party Rep Sun-min Kim (also a physician) is expected to reduce obstacles in upcoming committee reviews. Specifically, the bill requires the Ministry of Health and Welfare (MOHW) to establish a Supply Management Committee for Shortage Drugs. After deliberation and resolution by this committee, the Minister of Health and Welfare will designate shortage drugs. The committee will have up to 30 members, including the Vice Minister of Health and Welfare as Chair, Deputy Commissioner of the Ministry of Food and Drug Safety (MFDS) as Vice Chair. The remaining 28 will consist of : ▲Senior officials from relevant central government agencies (appointed by Presidential Decree), ▲ representatives recommended by the Chair of the Korean Pharmaceutical Association, ▲ representatives recommended by the Korean Medical Association under Article 28 of the Medical Service Act, ▲ representatives recommended by the Korea Pharmaceutical and Bio-Pharma Manufacturers Association and other relevant industry groups, as well as ▲ experts with sufficient knowledge and experience. Thus, government officials, pharmacists, physicians, manufacturers, and academia will collectively decide which drugs require mandatory generic prescribing due to unstable supply. The Minister of Health and Welfare will also have dedicated staff and budget authority to designate and de-designate shortage drugs, monitor supply conditions, and implement distribution improvement measures. The bill also legalizes distribution interventions alongside generic prescribing. If supply is deemed significantly disrupted, or upon request from another central administrative agency head, the Minister may—after committee review—order measures to improve distribution regarding sales outlets, procedures, volumes, and conditions. Pharmacies, medical institutions, wholesalers, and other designated entities will be legally obligated to comply with these measures. However, the Minister must first consult with the Minister of Strategy and Finance and the Chair of the Fair Trade Commission before issuing such orders. The MOHW Minister will also build and operate a shortage drug management system. This will allow requesting and collecting information necessary for distribution control, which includes production, shipments, sales, prescriptions, and dispensing data, from manufacturers, importers, wholesalers, pharmacies, and medical institutions. This effectively grants the MOHW authority over the entire supply chain, but also increases the Ministry’s accountability when shortages occur. The Minister may also designate certain shortage drugs as “emergency production/import drugs”, subject to committee review, which empowers the Minister to order manufacturers to produce or import the drugs. The Medical Service Act amendment stipulates that physicians and dentists must prescribe designated shortage drugs by generic name, not brand name. Violation will result in up to 5 years imprisonment or a fine up to KRW 50 million. This significant level of criminal penalty is expected to draw strong opposition from the Korean Medical Association and the broader medical community.
- Policy
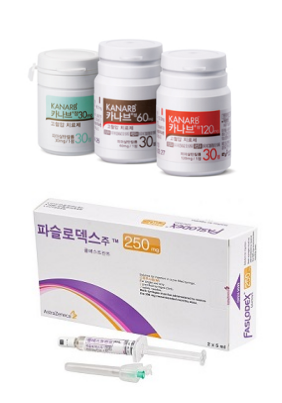
- Kanarb and Faslodex to keep their price until ruling
- by Lee, Tak-Sun Sep 02, 2025 06:11am
- The drug prices for Boryung's hypertension treatment ‘Kanarb’ and AstraZeneca's anticancer drug ‘Faslodex,’ which were determined to be reduced by the MOHW due to the entry of generics, will be maintained at their previous levels for now. The court has decided to suspend the execution of the price reduction until the first-instance ruling. As a result, the industry’s eyes are on whether these pharmaceutical companies will be able to halt the price reduction disposition through the main lawsuit. According to industry sources on the 1st, the Seoul Administrative Court accepted an application on the 27th of last month to suspend the execution of the price reduction order for 11 items, including Kanarb Tab. Consequently, the previous insurance price ceiling for the drugs will be maintained until two months after the date of the final judgment of the main trial. The affected items are Boryung's Kanarb Tab (3 dosages), Kanarb Plus Tab (2 dosages), Dukarb Tab (4 dosages), and Dongwha Pharmaceutical's LaCor Tab (2 dosages). At the end of June, the MOHW announced an ex officio adjustment and termination of the premium for these items following the entry of generic drugs containing the active ingredient, the single-ingredient fimasartan. Implementation was scheduled for July 1st. Four pharmaceutical companies' Kanarb generic products were listed for reimbursement last May. Consequently, health authorities proceeded with the ex officio price reduction process for Kanarb. Despite Boryung's objection, the price adjustment was ultimately finalized. The price caps for Kanarb Plus Tab and LaCor Tab, which are combination drugs that contain fimasartan, were also administratively reduced due to the price adjustment. For Dukarb, the premium granted for incrementally modified new drug combinations ended as two or more fimasartan-based single-ingredient drugs became available. Kanarb’s price was reduced by 30%, Dukarb by 21%, and Kanarb Plus and LaCor by 47%. Given that Kanarb and Dukarb each generate sales in the KRW 60 billion range, the price adjustment is expected to lead to a significant decrease in Boryung's overall sales performance. Consequently, many believed Boryung would seek to provisionally maintain its drug prices through litigation. The first trial for the main suit will commence in earnest with the first hearing scheduled for November 13. Kim & Chang is representing Boryung. The drug price for AstraZeneca's anticancer drug Faslodex (fulvestrant) will also be maintained until 30 days after the ruling date of the main case. With more than three companies producing generic versions, Faslodex's price adjustment period was scheduled to end last July. As a result, its insurance price cap was set to decrease from KRW 376,724 to KRW 288,194 starting August 1. AstraZeneca was reportedly considering withdrawing from the domestic market due to the Faslodex price cut disposition. This issue also surfaced during the confirmation hearing for Minister of MOHW Eun-Kyung Jeong. Domestic pharmaceutical companies have also expressed the view that if the original anticancer drug withdraws, it would be difficult for patients to find a substitute, given the market characteristics. Fortunately, the company did not withdraw its product but opted for litigation. On the 29th of last month, the Seoul Administrative Court granted its application for a stay of execution of the price reduction order. The main lawsuit was filed on July 25th, and no hearing date has been set yet. AstraZeneca's legal representative is the law firm Sejong. Although both pharmaceutical companies chose litigation to maintain drug prices, the risk is not entirely absent. This is because the Act on Drug Price Litigation Recovery and Refund that was implemented last year allows the National Health Insurance Service to refund the claimed amount for the trial period if the company loses the case. Nevertheless, analysis suggests the companies ultimately chose litigation due to the significant immediate financial loss and the potential for dispute over the price reduction decision.
- Policy
- "MFDS' data-based drug shortage response"
- by Lee, Jeong-Hwan Sep 02, 2025 06:09am
- Jeong Eun Keong, Minister of Health and Welfare The Ministry of Health and Welfare (MOHW) announced that it will address drug shortages by utilizing information from the "Pharmaceutical Production, Supply, and Prescription Data-based Supply Risk Prediction System," which is currently being established by the Ministry of Food and Drug Safety (MFDS). The MOHW has also proposed drug shortage response strategies, including domestically producing the raw materials and sub-materials for medicines and vaccines, as well as expanding a production support program for drugs with unstable supply to include up to four pharmaceutical companies. The MOHW also stated that the introduction of abortion drugs could only be expedited after the scope of legal abortion is defined through amendments to the Mother and Child Health Act and the Criminal Act. The following Q&A is based on the MOHW's responses to written inquiries from People Power Party Representative Han Ji-a and Democratic Party of Korea Representative Nam In-soon of the National Assembly's Health and Welfare Committee on August 27. "Responding to unstable supply by linking production, supply, and prescription systems" The MOHW's Division of Pharmaceutical Policy and Division of Health Industry Promotion responded to Rep. Han Ji-ah's inquiries about policy plans to strengthen the drug supply chain. First, the MOHW stated that "securing a stable drug supply chain is essential for protecting and maintaining public life and health, and is an essential task directly related to health security." The Ministry explained that it is "monitoring drugs with supply interruptions or shortages in cooperation with the MFDS, and taking customized measures through a public-private consultative body when necessary." The MOHW also stated that it would create countermeasures by linking its system with the one being developed by the MFDS for drugs with unstable supply. The MFDS is currently building a data-based drug supply risk prediction support system to enhance the prediction of unstable supply situations. The system is designed to comprehensively analyze drug production, supply, and prescription information. In particular, the National Assembly has a pending amendment to the Pharmaceutical Affairs Act that would strengthen the linkage of information on drugs with unstable supply between the MOHW and the MFDS. The Ministry expressed its commitment to support this legislation to address drug shortages actively. The National Assembly also promised to make efforts to produce drug and vaccine raw materials and sub-materials domestically, support the production of drugs with unstable supply, and stockpile essential medicines. The plan is to expand the project that provides budget support for pharmaceutical companies producing drugs with unstable supply from one to four companies. To respond to human infections of avian influenza and bioterrorism, the Ministry plans to prioritize the introduction of the latest overseas vaccines and stockpile them with domestically developed vaccines after their development is complete. It will also focus on national cooperation to diversify the sources of imports for active pharmaceutical ingredients (APIs) from China and India. "Introducing abortion drug requires an amendment to both Mother and Child Health Act and the Criminal Act" The MOHW states that amendments to the Mother and Child Health Act and the Criminal Act are essential for the domestic approval of abortion drugs like Mifepristone. It stated that to create a safe abortion environment, the scope of abortion must be established through amendments to the Criminal Act. While the Constitutional Court's ruling in April 2019 that the abortion ban was unconstitutional effectively nullified the relevant penal provisions, a legislative void has persisted for over six years due to the failure to pass supplementary legislation. As a result, the MOHW's stance is that it is difficult to carry out abortion-related administrative tasks. The MOHW plans to work closely with relevant ministries, including the Ministry of Justice and the MFDS, to support future legal and institutional improvements. Minister Jeong Eun Keong gave the same response during a questioning session with People Power Party Representative Cho Bae-sook at the National Assembly's Special Committee on Budget and Accounts on August 27. Minister Jeong explained, "As the Criminal Act and Mother and Child Health Act have not been amended since the unconstitutional ruling, there are safety issues." She added, "While there are no approved drugs in Korea, most countries in the world use approved drugs." And added, "The World Health Organization (WHO) also provides recommendations based on gestational age." Minister Jeong said, "I will consult with the MFDS on safe usage methods. We will consider both the fetus's right to life and the woman's right to health in a balanced manner."