- LOGIN
- MemberShip
- 2025-12-19 20:28:00
- Policy
- Daewoong wins nod for first generic version of Ibrance tab
- by Lee, Tak-Sun Oct 08, 2024 05:49am
- Daewoong's first generic for Pfizer's breast cancer treatment, 'Ibrance Tab,' has been approved. Due to the successful patent nullification, the company received priority marketing authorization. The first generic can be launched in March 23rd, 2027, when the product patent expires. The Ministry of Food and Drug Safety (MFDS) has granted approval of Daewoong's "Ranclib tab" 75 mg, 100 mg, and 125 mg. This drug can be used to treat hormone receptor (HR)-positive or HER2-negative metastatic or advanced breast cancer. It is used in combination with aromatase inhibitor as first-line endocrine therapy in women or in combination with fulvestrant after endocrine therapy in women with advanced diseases. PfizerPfizer's Ibrance containing palbociclib is the original medicine. Ibrance was the first CDK4/6 inhibitor to be developed. It was launched with reimbursement coverage in November 2017 through the risk-sharing agreement (RSA). Unlike capsules, tablets can be taken regardless of food. The film-coating of the tablet allows for co-administration with proton pump inhibitors (PPI) or antacids, which are used to manage gastrointestinal disorders or diarrhea that commonly occur in breast cancer patients. Overall, tablets provide easier administration convenience than capsules. Ibrance's 2023 sales were KRW 50.5 billion, according to IQVIA. Due to the drug's high marketability, Korean pharmaceutical companies are attempting at early market entries through patent challenges. In addition to Daewoong, Kwang Dong Pharmaceutical, Boryung, and Samyang have entered patent challenges. After winning the nullification trial for the ingredient patent in March, Daewoong has established a ground to launch generic once the substance patent expires. In addition to successfully winning the patent challenge, Daewoong also acquired priority marketing authorization with approval as it met the requirement for the first generic. The prior marketing authorization period is from March 23rd, 2027, the day after Ibrance Tab's substance patent expires, to December 22nd, 2027. Last year, Kwang Dong Pharmaceutical acquired the priority marketing authorization of 'Alency Cap' for the same period as Daewoong's product. However, Kwang Dong Pharmaceutical won the priority marketing authorization against Ibrance Cap rather than Ibrance Tab. Additional approvals are expected soon as other companies that have challenged the patent have won. At the launch, Ibrance had no competitors in the market for breast cancer. However, the sales are slightly decreasing as similar CDK4/6 inhibitors Kisqali (Novartis, ribociclib) and Verzenio (Lily, abemaciclib) have launched. The analysis suggests that the market size may be downsized upon generic launch. "Although Ibrance generates the highest sales in the CDK4/6 inhibitors market, the sales have been gradually decreasing," pharmaceutical industry personnel said. "Sales could drop even more when generics launch following the substance patent expiration. Korean pharmaceutical companies could have launched generics earlier."
- Policy
- Drugs for NSCLC harboring MET alterations pass CDRC review
- by Lee, Tak-Sun Oct 07, 2024 05:48am
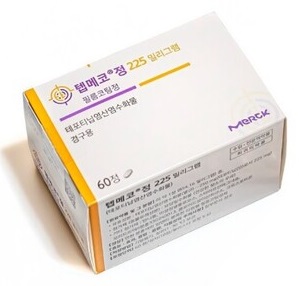
- Product photo of TepmetkoA treatment for non-small cell lung cancer (NSCLC) harboring MET alterations has passed the first stage for reimbursement coverage by national health insurance. Merk's Tepmetko is the drug that passed. On October 2nd, the Cancer Disease Review Committee (CDRC) of the Health Insurance Review and Assessment Service (HIRA) commenced the 7th meeting and set reimbursement standards for anticancer agents, including 'Tepmetko (tepotinib),' a treatment for patients with NSCLC harboring MET alterations. After receiving approval in November 2021, Tepmetko passed the CDRC review on the third attempt. It is the first treatment for NSCLC harboring MET alterations to pass the review in South Korea. The rate of patients with MET alterations in metastatic NSCLC is low, approximately 3-4%. However, due to poor prognosis, anticancer agents targeting such alterations are of paramount importance. In a clinical study, data showed that Tepmetko-treated stage IV patients with MET alterations had a median overall survival of 19.6 months, which was higher than the 13.4 months of immune checkpoint inhibitors. It became the new hope for patients. However, it repeatedly failed to set the reimbursement standards due to insufficiency in data utility. Other anticancer agents targeting MET alterations, such as Tabrecta, had similar results. Tepmetko successfully set the reimbursement standards in its fourth attempt, passing the first stages for receiving reimbursement. When it passes the Drug Reimbursement Evaluation Committee (DREC) of the HIRA, drug pricing negotiations with the National Health Insurance Service (NHIS), Tepmetko would be on the national health insurance reimbursement list. Review results for new drugs (applied for reimbursement decisions) and expanded reimbursement standards. Tepmetko (tepotinib, Merk) and Tibsovo Tab (ivosidenib, Servier Korea) successfully set reimbursement standards. Drugs that successfully expanded reimbursement standards include Jemperli (dostarlimab, GSK) and Neulasta Pre-filled Syringe (pegfilgrastim, Kyowa Kirin Korea). Along with Tepmetko, Tibsovo Tab (ivosidenib, Servier) successfully set reimbursement standards. The reimbursement standards for Tibsovo are set for use in combination with azacytidine in adult patients over 75 years with newly diagnosed locally advanced or metastatic acute myeloid leukemia (AML) who test positive for isocitrate dehydrogenase-1 (IDH1) mutation or those with accompanying disease who cannot receive chemotherapy. Additionally, Jemperli (dostarlimab, GSK) and Neulasta Pre-filled Syringe (pegfilgrastim), which have applied for expanded reimbursement, were successful in expanding the standards. Jemperli now has established reimbursement standards for use in combination with platinum-based chemotherapy in adult patients with newly diagnosed advanced or relapsed mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) endometrial cancer. Neulasta successfully set reimbursement standard for patients receiving cytotoxic chemotherapy for malignant tumors to reduce the occurrence and duration of neutropenia. In contrast, Verzenio failed to establish expanded reimbursement for adjuvant therapy in combination with endocrine therapy for adult patients with early-stage breast cancer who are hormone receptor (HR)-positive or human epidermal growth factor receptor 2 (HER2)-negative or who are likely to have relapses of lymph nodes. The CDRC has established a policy for reviewing the reimbursement coverage of an existing drug when newly added high-price drugs are to be used in combination with a drug that is already covered by reimbursement. Medical organizations, including doctors' associations and hospital associations, requested such improvement last year.
- Policy
- 'Qarziba' to be reconsidered for the DREC review in KOR
- by Lee, Tak-Sun Oct 07, 2024 05:48am
- Product photo of Qarziba (dinutuximab, Recordati Korea).Qarziba (dinutuximab, Recordati Korea), which was designated as the 1st drug for a "Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations" will be reconsidered for the Drug Reimbursement Evaluation Committee (DREC) review. Qarziba was considered for the DREC review in August but received a non-reimbursement decision. Then, the company applied for re-evaluation. According to industry sources on October 4th, Qarziba has been selected as the agenda item for the 10th DREC meeting, which will be held on October 10th. Qarziba has drawn industry attention as the 1st drug for a "Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations." The project conducts the Ministry of Food and Drug Safety (MFDS) approval, drug evaluation, and drug pricing simultaneously, expediting the insurance listing process, including approval, drug evaluation, and the Ministry of Health and Welfare (MOHW) reporting. The drug successfully set reimbursement standards before receiving marketing authorization through this designation. On May 29th, the 4th CDRC meeting set the reimbursement standard for this drug for young children over 12 months with ▲A high-risk neuroblastoma who had received bone marrow removal therapy and a stem cell transplantation therapy after showing partial adverse reactions following chemotherapy ▲Recurrent and refractory neuroblastoma. Then, on June 19th, it received the official marketing authorization from the MFDS. The approval review period was shortened from 115 days to 90 days after it was granted a designation as the Global Innovative products on Fast Track (GIFT). Despite the expectation for a speedy reimbursement approval, Qarziba was halted at the DREC review, which decides reimbursement appropriateness. On the 8th DREC review, held on August 8th, Qarziba received a non-reimbursement decision for treating neuroblastoma in children. After receiving the DREC's non-reimbursement decision, the company applied for re-evaluation from the Health Insurance Review and Assessment Service (HIRA). The policy states that a company can apply for re-evaluation within 30 days of receiving a DREC review report. The HIRA re-evaluated based on the application, and Qarziba is set to receive a DREC review again. If Qarziba were to receive a non-reimbursement decision, the purpose of the "Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations" to support expediting reimbursement listing would not be met. If Qarziba receives a reimbursement appropriateness decision this time, it would have taken a year and a half since the approval application. Typically, the process from approval application to reimbursement coverage takes at least three years, but Qarziba would have cut that time in half. Qarziba was designated as the 1st drug for a "Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations" in June, and it has been on a track for registration in South Korea. "Qarziba will likely be re-evaluated for this DREC review," sources said, adding, "If it receives reimbursement appropriateness decision, it will also be on an expedited track for the negotiation with the National Health Insurance Service (NHIS) due to the pilot project. It may be considered for the Health Insurance Policy Review Committee within this year."
- Policy
- EUA for Novavax's COIVD-19 vaccine targeting the variant
- by Lee, Hye-Kyung Oct 02, 2024 05:49am
- The Ministry of Food and Drug Safety (Minister Oh Yu-kyoung, MFDS) announced emergency use authorization of Novavax's 'COVID-19 Vaccine corresponding to JN.1 strain of SARS-CoV-2 (2024-2025 Formula).' The emergency use authorization is a system designed to respond appropriately to a public health crisis, including infectious disease pandemics. In this system, the MFDS minister enables the supply of medical products that do not have domestic approval by ordering manufacturers and importers to manufacture or import upon requests from the central administrative agency. MFDS gathers expert opinions by reviewing companies' submitted clinical and quality documents through the official posting of the emergency use authorization upon request from the central administrative agency. Then, after review and voting from the committee for the 'Public Health Emergency Preparedness and Responses for a Medicinal Product,' the MFDS makes a final decision. The Korea Disease Control and Prevention Agency (KDCA) requested the emergency use authorization based on the vaccination plan to prevent COVID-19 for this winter. The MFDS quickly reviewed the item and approved of it. SK Chemicals will be responsible for importing and distributing the Novavax vaccine. Novavax vaccine is a direct injection of antigen protein using a recombinant protein vaccine technology to induce the synthesis of vaccine-eliminating antigens. With the Novavax COVID-19 vaccine entering South Korea, clinical fields will have wider array of choices for vaccine types. Recently approved Pfizer vaccine and Moderna vaccine are made of mRNA, which is designed to express antigen protein that induces immune responses in the body. The MFDS stated it would provide safe vaccination for people by strengthening the safety management system for COVID-19 vaccines, including managing quality and collecting reporting of adverse reaction cases.
- Policy
- "Hundreds of doctors have received pharmaceutical rebates"
- by Kang, Shin-Kook Sep 30, 2024 05:47am
- Min Juwon, Director of Audit at the National Tax Service (NTS).After conducting a tax audit of 16 pharmaceutical companies, Korea's National Tax Service (NTS) announced that it would impose an income tax for doctors who received rebates. The NTS mentioned that over hundreds of doctors received rebates, and rebate-associated medical-pharmaceutical cartels became a significant issue. As a result, the NTS will continue to conduct intense tax audits. The following article reconstructs the briefing by Min Juwon, Director of NTS Audit, held on Septebmer 2nd in a question-and-answer format. - Min said the current audit focuses on imposing income tax on medical professionals who had received rebates. We impose income taxes on doctors who received rebates in any form. Because the customary way of rebates between the medical and pharmaceutical industries has continued for an extended period, the NTS has been paying attention and continuously conducting tax audits. During previous tax audits, we tried identifying the final recipients of rebates through pharmaceutical companies, but we could not confirm the allegations by solely relying on hearsay. However, this round of tax audits focused on eradicating rebates by imposing taxes on individuals who received rebates. - How were the companies selected for audits? We have not focused on whether the audited pharmaceutical company has been prosecuted by the Fair Trade Commission (FTC). The NTS selected companies that have internal issues. Once audits are completed, we plan to decide whether we will share audit outcomes with other agencies, according to regulations. Regarding the rebates being investigated by the prosecution and the Korean National Police Agency, we expect them to cooperate with the NTS regarding tax issues. When we receive documentation, we plan to investigate tax issues and impose on it thoroughly. -Some argue that the tax audit has a political agenda amid the conflict between the medical community and the government. Before today's announcement, we were aware of the concern. According to the law, the NTS conducts audits within the National Tax Imposition Exclusion Period, defined as the past five years. The NTS conducts audits of allegations within the designated period. Moreover, individuals who are involved in medical conflicts are not being audited. Audits are being conducted in individuals across the nation, including primary, secondary, and tertiary medical centers. We want people to know that the NTS focuses on rebates rather than the current issues related to the medical community. The conflict between the medical community and the government is recent. Auditing takes time because companies have to report, the NFS analyzes them, and commences on audits. Consequently, we are focusing on records dating 3-4 years back. For this audit, we have not collaborated with the Ministry of Health and Welfare (MOHW) in any form. We have conducted the audit based on NTS criteria for selecting auditing subjects and identified the degree of tax invasion. - How many medical professionals have received rebate? Subjecting companies that are being audited, hundreds of medical professionals have received rebates. If we continue, the number is expected to increase. - Min stated the medical-pharmaceutical cartel is a grave problem. Medical professionals are of superior status to pharmaceutical companies. When a certain pharmaceutical company names rebate recipients, it will no longer be able to operate in South Korea. As a result, we used the word, "medical cartel." It is a grave problem.
- Policy
- Stelara biosimilar Steqeyma IV approved for use in Korea
- by Lee, Hye-Kyung Sep 30, 2024 05:46am
- Upon receiving approval for ‘Steqeyma IV,’ a biosimilar version of the autoimmune disease treatment Stelara (ustekinumab), Celltion has now received approval for all its Steqeyma formulations. By adding the interleukin (IL) inhibitor option to the existing family of tumor necrosis factor (TNF)-α inhibitors, which includes Remsima, Remsima SC, and Yuflyma, the company has strengthened its autoimmune disease portfolio. On the 27th, the Ministry of Food and Drug Safety (MFDS) approved Celltrion's Steqeyma IV. Celltrion received approval for the prefilled syringe-type subcutaneous (SC) formulation of the same drug in June for the treatment of autoimmune diseases such as plaque psoriasis, psoriatic arthritis, Crohn's disease (CD), and ulcerative colitis (UC) With the approval of the IV formulation, the company can now compete with the original with the same formulation. Earlier, in April, Samsung Bioepis first received approval for 2 Stellara biosimilars – Epyztek IV and Epyztek SC. The insurance drug prices of Steqeyma and Epyztek were set at the same level - about 26% lower than the original - being priced at KRW 1,298,290 for the 45 mg formulation and KRW 1,342,320 for the 90mg formulation, respectively. The original, Stelara, is a global blockbuster drug developed by Janssen, whose product patent expired in July this year in Europe and last year in the United States. According to the drug market research institution IQVIA, the global ustekinumab market was valued at around KRW 26.42 trillion last year. Starting with the domestic marketing authorization of Steqeyma, the company plans to acquire approvals in major global countries such as the U.S. and Europe and target the global ustekinumab market in earnest. Meanwhile, Samsung Bioepis was the first to obtain marketing authorization for a Stelara biosimilar in Korea, and in addition to Celltrion, Dong-A ST (DMB-3115) is also developing a biosimilar of Stelara.
- Policy
- COVID-19 drugs to be reimbursable, "KRW 50,000 co-payment"
- by Lee, Jeong-Hwan Sep 27, 2024 05:53am
- The National Health Insurance will cover two COVID-19 treatments, Paxlovid and Veklury, starting on October 1st. The government will list COVID-19 treatments for reimbursement and make a policy revision to support the patient co-payment at the previous price of KRW 50,000. The National Health Insurance coverage for Takeda Pharmaceuticals Korea's Zejula Cap (ingredient: niraparib tosylate monohydrate), a treatment for advanced ovarian cancer, will be expended, and the ceiling price of the drug will be reduced, starting on October 1st. Patients with advanced ovarian cancer who paid KRW 41 million in annual treatment costs per person are expected to pay KRW 2..05 million in annual treatment costs with the National Health Insurance coverage. On September 26th, the Ministry of Health and Welfare (MOHW) held the 19th Health Insurance Policy Review Committee meeting and decided on items related to National Health Insurance coverage of COVID-19 treatments and expanded National Health Insurance coverage for Zejula Cap. COVID-19 drugs will be covered by NHI…expanded reimbursement for Zejula The two COVID-19 treatments that the Korea Disease Control and Prevention Agency (KDCA) purchased and supplied will be covered by the National Health Insurance, starting in October. Those two are Paxlovid and Veklury. The ceiling price for Pfizer Korea's 'Paxlovid' is KRW 941,940 per 30 tablets in a package, and that for Gilead Sciences Korea's 'Veklury 100mg Powder For Concentrate For Solution For Infusion' is KRW 520,000 per vial. The related policy will be revised along with the reimbursement listing of these drugs. As an administrative action to prevent patient co-payment increases due to reimbursement, the co-payment will be maintained at the current cost of KRW 50,000. Starting next month, the reimbursement coverage for Zejula Cap, a treatment for advanced ovarian cancer, fallopian tube cancer, and primary peritoneal cancer, will be expanded, and its ceiling price will be reduced. For Zejula Cap, the usage criteria will be expanded, and it will be reimbursable for maintenance therapy of patients with advanced epithelial ovarian cancer, fallopian tube cancer, and peritoneal cancer who are responsive to the first-line platinum-based therapy. Previously, it was reimbursable only when the patients tested BRCA mutation-positive ovarian cancer from genetic screening. Starting next month, the drug will also be reimbursable in all homologous recombination deficiency-positive genetic mutations, including ovarian cancer-associated genomic instability identified from genetic testing. Patients with advanced ovarian cancer who paid KRW 41 million in annual treatment costs per person are expected to pay KRW 2..05 million in annual treatment costs with the National Health Insurance coverage. The pilot project for primary healthcare home visits will be improved Since December 2019, the government has been implementing a pilot project for primary healthcare home visits, a healthcare service in which private clinic doctors visit patient homes, to enhance healthcare access for patients who have difficulties visiting medical centers. Starting in November, the designation of medical centers for healthcare home visits will be expanded to include hospitals (local medical centers). Peviously, only private clinics and oriental medicine clinics were eligible for designation. Now, hospitals (local medical centers) can also participate following the policy revision. It will be expanded for patients with severe disease who require home medical care, including bedridden patients with Grade 1·2 long-term care and patients with severe disease using medical devices (oxygen therapy and a respirator). Co-patients for home visits will be reduced for these patients. In order to implement a reduction in out-of-pocket costs for eligible patients, a medical center's screening process will be developed. Additionally, a computer system for patients to claim the reduction of out-of-pocket costs is also in development. The implementation of the out-of-pocket cost reduction is scheduled to occur after November. In addition, to expand the primary healthcare home visit project, additional participating institutions will be invited in October. Extended NHI support for emergency care The policy for providing the National Health Insurance coverage for emergency care, which costs KRW 208.5 billion per month, will be extended to prevent a gap in healthcare for patients with severe and emergent symptoms amid the doctor's strike. The budget will be used to strengthen compensation for emergency departments and tertiary general hospitals so that they can focus on the treatment of emergency and patients with severe symptoms, and for the return of patients with mild symptoms to hospitals and clinics. Incentives will be increased to ensure the prompt transport of critical patients to hospitals. Additionally, incentives for medical care in emergency centers, including emergency care fees and CPR fees, will be enhanced. To facilitate prompt hospital responses to critical patient emergencies, the government will provide financial support for specialists treating hospitalized critical patients and incentives for critical patient hospitalization. The emergency medical care analysis comparing year-over-year from March to July 2024 shows that hospitals are maintaining critical patient care systems. However, the number of critical patients visiting the regional·local emergency medical centers has slightly decreased. The government will extend additional support to strengthen emergency care treatment capacity. The government will also extend raises for specialist medical fees, which were temporarily implemented during Chuseok to maintain the infrastructure of critical·emergency patient treatment at emergency medical centers. The raises for critical·emergency surgeries will also be extended. The raises in specialist medical fees will be 250% for regional·special emergency medical centers and 150% for local emergency medical centers. The raises for critical·emergency surgeries at regional·special·local emergency medical centers will be 200%. The MOHW stated, "We will provide a temporary insurance fee for emergency medical care to prevent a medical care gap for emergency and critical patients. We aim to promptly solve the current situation to ensure people do not encounter difficulties using medical centers."
- Policy
- 2nd Approval-Evaluation-Negotiation pilot project imminent
- by Kim, Jin-Gu Sep 26, 2024 05:51am
- The initiation of the 2nd pilot project for the ‘approval-evaluation-negotiation linkage system,’ which was introduced to shorten the time from drug approval to reimbursement, is imminent. The Ministry of Health and Welfare has completed receiving drug submissions for the 2nd pilot project from pharmaceutical companies and is in the final review stage. The MOHW plans to implement the 2nd pilot project after reflecting on the improvements identified during the 1st pilot project. Eun-Joo Lee, an official from the Ministry of Health and Welfare’s Division of Pharmaceutical Benefits, announced so regarding the pilot project linking the approval-evaluation-negotiation system at the ‘Roundtable on Improving Access to Rare Disease Drugs' that was held at the National Assembly on the 24th. The government implemented the 1st pilot project for the approval-evaluation-negotiation linkage system last year. The project consists of simultaneous approval by the MFDS, reimbursement evaluation by the Health Insurance Review and Assessment Service, and drug price negotiations by the National Health Insurance Service. The project shortens the 300-day review period required for new drug access in Korea – which consists of an MFDS approval period of 120 days, 150 days of reimbursement evaluation by HIRA, and 60 days of drug price negotiations by the NHIS - and strengthens Korea’s access to new drugs. Two drugs - Qarziba Inj (dinutuximab, Recordati) and Bylvay (odevixibat, Ipsen) – were selected for the 1st pilot project. At the time, the government proposed the following criteria for selection: ▲a drug for cancer or rare diseases with a life expectancy of less than one year; ▲has a small number of patients; ▲lacks alternative drugs; ▲has a survival period over two years and demonstrated a superior treatment effect. Several companies submitted applications to participate in the pilot program, among which the government ultimately selected 2 drugs that met the selection criteria. Although the 1st pilot project has not been completed yet, the Ministry of Health and Welfare has been promoting to launch of the second pilot project. “We have completed receiving applications for the 2nd pilot project. We now need to select the drugs that meet the conditions of the 2nd pilot project,” said Lee. He added, “The number of drugs for the 2nd pilot project has not been decided upon yet. There are no criteria that require us to select only 2 drugs as in the 1st project. We will select drugs that meet the conditions.” The Ministry of Health and Welfare has proposed 4 criteria for selecting drugs for the 2nd pilot project: ▲ drugs that can apply for approval and decision by the end of June next year; ▲ drugs with sufficient effect for the treatment of life-threatening diseases (life expectancy of less than 1 year) or rare diseases; ▲ drugs that have no existing treatment or have shown clinically significant improvements in efficacy over existing treatments; and ▲ drugs that have been designated or are eligible for Global Innovative products on Fast Track (GIFT). To be selected as a drug for the 2nd pilot project, the drug needs to satisfy all the conditions above, and the company needs to submit the following data: ▲estimated financial expenditure per capita and total estimated financial expenditure; ▲results of overseas reimbursement evaluations; ▲the number of listed overseas countries; ▲the name of listed countries; and ▲name of the company. The MOHW plans to analyze the pros and cons of the 1st pilot project and apply them to the 2nd pilot project. In the 1stpilot project, the approval and reimbursement evaluation processes for Qarziba have been completed. The agenda had passed the Drug Reimbursement Evaluation Committee review and is currently being reviewed upon the company's reapplication. Compared to the existing system, the time from its approval to reimbursement evaluation has been significantly reduced. However, in the case of Bylvay, there are some delays due to difficulties in the evaluation process. The marketing authorization was completed on the 23rd of last month, but the reimbursement evaluation process was hampered due to the use of a difficult evaluation tool. However, the government is focusing on shortening the timeframe by conducting pre-negotiations at the same time. The HIRA explained that it will focus on how the companies manage uncertainties during the reimbursement evaluation process in the 2nd pilot project “In the future, uncertainty management will be important in the reimbursement evaluation process,” said Kook Hee Kim, Director of the Pharmaceutical Benefits Management Division at HIRA. ”Since the drugs are expected to be effective but not yet certain, the pharmaceutical companies should discuss how to address the uncertainties and prove their drug’s effect in the post-marketing process after the fact and share the risk with the government.” “The approval-evaluation-negotiation process is not a guarantee that the drug will be reimbursed. It means that the evaluation period is shortened,” explained Kim. ”The companies should submit data on how they will prove the uncertainties in the post-marketing process. Otherwise, the drug’s reimbursement may be delayed.”
- Policy
- Higher dosage Eylea expected to strike back at biosimilars
- by Lee, Tak-Sun Sep 26, 2024 05:51am
- The competition in the market for the macular degeneration drug Eylea has recently intensified with Samsung Bioepis and Celltrion, which have recently launched biosimilars. The original developer is set to strike back against the competitors. Bayer will release Eylea Inj 8 mg (aflibercept), with an extended treatment interval, into the market next month at a relatively cheaper price. According to the industry on September 25th, Eylea Inj 8 mg will be listed for reimbursement next month at a price lower than the evaluation price, KRW 795,000. Eylea Inj 8 mg provides a long-lasting, effective dosage within the eye at a four times higher dosage than the previous 2 mg. It extends the treatment interval while reducing the number of injections. Eylea Inj 8 mg is injected once monthly for an initial 3 months. Then, based on visual and anatomical test outcomes, a treatment interval can be extened up to 16 weeks at the physician's judgement. After the initial extension of the treatment interval, it can be extended up to 20 weeks with the Treat-and-Extend(T&E) therapy. This approach allows flexibility in adjusting the treatment interval depending on the patient's condition while maintaining stable vision and anatomical test outcomes. The original and biosimilars currently sold in the market are at a 2 mg dosage. For neovascular age-related macular degeneration (nAMD) treatment, the drugs are injected once monthly for an initial 3 months, followed by a once every 2 months treatment regimen. Using the new 8 mg will extend the treatment interval more than twice, from once every 2 months to once every 4 months. The PULSAR study secured non-inferiority results of the new product by showing that patients treated with Eylea 8 mg at a 12-week interval had the best corrected visual acuity (BCVA) of 6.7 letters on average from the baseline at 48 weeks, those at a 16-week interval had BCVA of 6.2 letters, and those at an 8-week interval had the BCVA of 7.6 letters. However, the drug price is not more than double the original price. Due to the biosimilar release, the current ceiling price for Eylea Inj·Prefilled Syringe is KRW 496,118, a 30% reduction from the highest price. The newly listing Eylea Inj 8 mg costs KRW 795,000. Despite extending the treatment interval more than twice, the price has not doubled because Bayer has set the price lower than the evaluated price. However, the price is double that of biosimilars. Samsung Bioepis' Afilivu Inj 40 mg/ml costs KRW 350,000, and Celltrion's Eydenzelt Inj costs KRW 330,000. Although the price of biosimilars is low, newly diagnosed patients are likely to choose the high-dose original drugs, which require fewer injections due to the extended treatment interval. Hence, Eylea Inj 8 mg will likely be a direct competitor to biosimilars. Eylea is a blockbuster product, recording KRW 96.8 billion in sales last year, based on IQVIA. Due to its marketability, Samsung Bioepis and Celltrion, which have released their products in May and September, respectively, are trying to seize the market by partnering with pharmacists who are specialists in ophthalmology. Industry personnel said, "Patients who are benefiting from the existing Eylea 2 mg are less likely to switch products. However, new patients are likely to choose from biosimilars, with lower cost, and high-dosage original drug, with fewer injections," adding, "An intense competition among overseas original developer and Korean biosimilar developers is expected."
- Policy
- Potential expanded indication for obestiy drug 'Wegovy'
- by Lee, Hye-Kyung Sep 26, 2024 05:51am
- Product photo of Wegovy. A clinical trial for Novo Nordisk Pharma's obesity drug, 'Wegovy (semaglutide),' will be conducted in South Korea for expanded indication. On September 20th, the Ministry of Food and Drug Safety (MFDS) has approved the 'Phase 2 clinical trial to evaluate the efficacy, safety, and dosage testing of NNC0519-0130, subcutaneously administered once-weekly, in comparison to Ozempic (semagluide) 1.0 mg and placebo in overweight or obese patients who have or do not have type 2 diabetes.' The clinical trial will involve 456 individuals worldwide from December 2024 to September 2026. In South Korea, the study will enroll 32 patients and will be conducted at Pusan National University Hospital, Ilsan Hospital, Hallym University Medical Center, Seoul St. Mary's Hospital, Hanyang University Guri Hospital, and Korea University Guro Hospital. Wegovy received domestic marketing authorization in April with indications to ▲aid weight-loss overweight patients who have a Body Mass Index (hereafter referred to as BMI) of 30kg/m2 or higher or those who are overweight with early BMI of 27kg/m2 or higher and below 30kg/m2 and having one or more weight-related accompanying diseases (abnormal glycemia, type 2 diabetes, high blood pressure, dyslipidemia, obstructive sleep apnea, or cardiovascular diseases) ▲reduce risks of major cardiovascular events in overweight or obese patients with early BMI of 27 kg/m² or higher with cardiovascular diseases. Additionally, the company will conduct the Phase 2 clinical trial to add indication to treat chronic kidney disease. Based on Phase 3 SELECT study results, presented by Dr. Katherine Turtle, a professor at the University of Washington School of Medicine, at a recent European Renal Association meeting, Wegovy can reduce the risk of kidney failure and death in patients with chronic kidney disease because of type 2 diabetes. The clinical trial involved 3533 patients with type 2 diabetes and chronic kidney disease. Half of the participants received weekly semaglutide injections for 3 years, and the rest received weekly placebo. Patients treated with semaglutide had a lower possibility of worsening symptoms that require dialysis or kidney transplant by 24% and a lower possibility of death due to major cardiovascular disease, including heart attack, than those treated with placebo by 29%. Patients treated with semaglutide had a 20% lower possibility of death of all causes during the study period. However, investigators studying semaglutide have not found an exact mechanism by which this drug helps the kidney, and the study was limited in that around two-thirds of the participants were white males. Meanwhile, Wegovy's active ingredient, semaglutide, is a 'Glucagon-like protein (GLP)-1' agonist. GLP-1 receptors lower blood pressure and regulate appetites. The basis for Wegovy's approval in South Korea was the STEP study, in which patients treated with Wegovy (1306 patients) had a 14.9% weight loss across 68 weeks compared to the baseline, showing a significant difference from a 2.4% of the placebo group (655 patients). According to Novo Nordisk Pharma's business performance reporting for the first half of 2024, Wegovy generated US$3.07 billion, up 74% year-over-year.