- LOGIN
- MemberShip
- 2025-12-18 02:39:58
- Company
- New pulmonary hypertension drugs are competing for approval
- by Moon, sung-ho Aug 11, 2025 06:03am
- Global pharmaceutical companies are introducing new treatment options for pulmonary hypertension, utilizing novel mechanisms, which are being successively approved in the Korean market. These drugs are expected to change the clinical treatment paradigm. It's anticipated that they will establish a new market alongside the currently reimbursed treatments. According to the pharmaceutical industry source on July 28, the Ministry of Food and Drug Safety (MFDS) recently approved Winrevair (sotatercept) from MSD Korea and Opsynvi Tab (macitentan-tadalafil) from Janssen Korea as treatments for pulmonary hypertension. Pulmonary hypertension is a rare, severe, intractable disease where the walls of the pulmonary arterioles abnormally thicken and narrow, causing pressure to rise. As the disease progresses, symptoms like shortness of breath, chest pain, and fainting appear, severely limiting all aspects of daily life. Additionally, the increased pressure in the right ventricle due to the narrowed pulmonary arteries gradually weakens the function of the right side of the heart, leading to a high risk of sudden death. First, MSD Korea's Winrevair is an 'Activin Signaling Inhibitor (ASI), the first-in-class (as of July 2025)' to be approved in the field of pulmonary hypertension. It is a new treatment mechanism that has emerged after 20 years. Its mechanism directly addresses the fundamental cause of the disease by blocking the excessive proliferative signals of the protein complex 'activin,' which causes cell proliferation within pulmonary artery blood vessels, and by restoring the balance with anti-proliferative signals that inhibit cell growth, thereby inducing reverse remodeling to normalize the deformed vascular structure. MFDS approved Winrevair for use in combination with existing treatments to improve the exercise capacity of adult patients (18 years or older) with pulmonary hypertension (WHO Group I) who are in WHO Functional Class II-III. The three existing treatments are endothelin receptor antagonists (ERA), PDE-5 inhibitors (PDE-5i), and prostacyclin analogues (PCA). With the addition of this new mechanism, the Activin Signaling Inhibitor, which differs from existing treatments, has further broadened the options for pulmonary hypertension patients. The STELLAR clinical trial, which served as the basis for approval, evaluated the efficacy and safety of Winrevair in 323 adult patients with pulmonary hypertension in WHO-FC II or III. During the 24-week study period, patients received either Winrevair or a placebo in combination with their existing treatment once every three weeks. Winrevair increased the 6-minute walk distance by 40.8m compared to placebo at the 24-week mark (95% CI, 27.5-54.1; P
- Company
- Jaqbo’s substance patent extended to 2040
- by Kim, Jin-Gu Aug 08, 2025 06:03am
- Onconic Therapeutics announced on the 7th that the patent term for Jaqbo (zastaprazan), a new drug for gastroesophageal reflux disease in the P-CAB (potassium-competitive acid blocker class, has been extended by four years and two months. According to the company, the Korean Intellectual Property Office recently extended the patent term of Jaqbo’s substance patent, “Imidazo[1,2-a]pyridine derivatives, methods for preparing the same and use thereof” from July 5, 2036, to September 13, 2040, for approximately 4 years and 2 months. The KIPO recently made an official decision on the extension registration and published it in the official gazette. The patent term extension registration system is designed to compensate for the reduction in the actual patent term due to issues such as the time taken for marketing authorizations. If certain requirements are met, the patent term can be extended for up to five years, which is considered a key mechanism for protecting the intellectual property of new drug development companies and securing market competitiveness. Jaqbo Onconic Therapeutics’ new drug, which was approved in April last year as the 37th domestically developed new drug. It is a P-CAB gastric acid secretion inhibitor and is evaluated to have faster efficacy and superior nighttime gastric acid control compared to existing PPI (proton pump inhibitor) type treatments. Recently, it has been approved for use in treating not only gastroesophageal reflux disease but also gastric ulcers. Jaqbo’s sales surpassed KRW 10 billion in quarterly prescriptions less than a year after its launch in October last year. The company expects that the patent extension will accelerate sales growth in line with its strategy to expand the indications for Jaqbo and advance into global markets. An official from Onconic Therapeutics said, “With this patent extension, Jaqbo will be able to maintain its exclusive position in the domestic market until 2040,” adding, “As the rights to our independently developed new drug have been strengthened, we plan to focus our capabilities on the development of subsequent pipelines.”
- Company
- Rapid growth in RSV prevention market for infants
- by Whang, byung-woo Aug 08, 2025 06:02am
- After the COVID-19 pandemic, the importance of preventing infectious diseases has been re-emphasized. A new anti-RSV (Respiratory Syncytial Virus) antibody injection for infants, Beyfortus, is gaining attention as a potential turning point in the vaccine market Korea. Attention has been focused on whether a presidential pledge to support this antibody injection, which marks a global shift in RSV prevention strategy away from only high-risk groups, will lead to its inclusion in the National Immunization Program (NIP). According to the '2024 Vaccine Industry Trend Report' published by the Ministry of Food and Drug Safety (MFDS), the global vaccine market size in 2023 was approximately $34 billion, showing a steep growth from 2022 and marking the most significant increase in the last five years. Analysis suggests that the primary reason for this is attributed to the increased focus on the entire vaccine industry, as infectious disease response capabilities emerge as a national competitive asset following the COVID-19 pandemic. The RSV prevention options market, in particular, is experiencing significant growth. The two RSV prevention options recently launched in Korea are Sanofi's 'Beyfortus' and GSK's 'Arexvy.' Arexvy can be administered to adults aged 60 and over, while Beyfortus is for all newborns and infants entering their first RSV season. Children up to 24 months of age who are at high risk for severe RSV disease during their second RSV season can also receive it. The MFDS report projects that the RSV market, which began with approvals in 2023, will grow rapidly to $4.5 billion to $ 7.5 billion by 2027. However, the growth trajectory is expected to vary slightly depending on the indication, for instance, pediatric vs. adult. Due to factors such as adjusted recommended ages in the U.S., Arexvy's cumulative global sales decreased by 37% year-over-year by Q3 2024. In contrast, Beyfortus recorded cumulative sales of approximately KRW 1.2609 trillion during the same period, representing a 516% year-over-year increase. This growth is interpreted as a significant expansion in demand for infant and newborn RSV prevention options, as their immature bronchi and lungs can worsen to bronchiolitis or pneumonia, leading to hospitalization. RSV is a common respiratory virus that infects 90% of infants and young children under the age of two. It's a leading cause of hospitalization for respiratory infections in this age group during late autumn and winter, requiring special attention from families with young children. The issue is the lack of a clear prevention option for RSV infection until recently. Before Beyfortus was launched, an antibody injection called Synagis was only used for high-risk infants and young children, such as those who were premature or had underlying conditions like heart or lung disease. However, persistent criticism has been raised about the existence of a preventive blind spot, as approximately 80% of infants who visited hospitals for RSV were healthy full-term babies with no underlying conditions. Consequently, there has been a high unmet need for a preventive option for all newborns and infants. The introduction of the RSV preventive antibody injection, Beyfortus, has started to address these unmet needs. Launched in Korea in February of this year, Beyfortus is an RSV preventive antibody injection that can be administered to all newborns and infants entering their first RSV season, as well as high-risk children under 24 months. Since it provides continuous protection for at least five months with a single injection, either before or during the season, garnering significant interest from parents of infants and young children since its launch. Dr. Ki Wook Yun, a professor in the infectious diseases division of the Department of Pediatrics at Seoul National University College of Medicine, explained, "RSV is a virus that infects 90% of infants and young children under the age of two, and in some infants, symptoms can worsen to bronchiolitis or pneumonia, leading to hospitalization." This response is also confirmed in the global market. In places like Galicia, Spain, and Queensland, Australia, Beyfortus has been included in their National Immunization Programs (NIPs) and regional immunization programs, leading to a reduction in RSV-related hospitalizations. Notably, in Queensland, Australia, the importance of vaccination is being re-emphasized, as evidenced by the fact that there were no RSV hospitalizations in infants who received Beyfortus over a four-week period. In Korea, however, the Beyfortus vaccination is paid for entirely out of pocket. Although it's a preventive option for RSV, which is known to infect two out of three infants under one year old, its cost burden means only a few families can afford it. This raises concerns about health equity. However, the current administration's presidential pledge included support for an anti-RSV antibody injection for infants and young children, which has led to growing expectations for future policy changes. If this policy is implemented through measures such as including Beyfortus in the NIP, all newborns and infants in Korea will be able to receive Beyfortus without incurring the cost burden. In this regard, Yun commented, "Although only out of pocket vaccination is currently possible in Korea, I hope that support for the anti-RSV antibody injection will be expanded as part of the government's low birth rate and infant health policies."
- Company
- Immunosuppressant market rapidly expands in 3yrs in KOR
- by Kim, Jin-Gu Aug 08, 2025 06:02am
- The market for major immunosuppressants such as tacrolimus, cyclosporine, and mycophenolate has rapidly expanded over the past three years. The related market grew relatively slowly at less than 5% until 2022, but since 2023, it has expanded by more than 10% a year, showing a sharp growth trend. The tacrolimus and mycophenolate markets also showed continued growth in the first half of this year. By company, Chong Kun Dang’s performance is standing out in all three ingredients. In the cyclosporine and mycophenolate markets, Chong Kun Dang’s Cipol-N and My-Rept are dominating with over half of the market share. In the tacrolimus market, the company has been ranking second. The once-dormant tacrolimus immunosuppressant market grows rapidly from 2023 According to a report by the pharmaceutical market research institution UBIST on the 8th, the outpatient prescription sales of tacrolimus-containing immunosuppressants in the first half of this year amounted to KRW 34.9 billion. This represents a 10% increase from the KRW 31.7 billion in the first half of last year. Tacrolimus is a calcineurin inhibitor class immunosuppressant. It is primarily used to suppress rejection reactions after liver, kidney, and bone marrow transplants, as well as to treat autoimmune diseases such as rheumatoid arthritis and lupus nephritis. In the outpatient prescription market, prescriptions for the treatment of autoimmune diseases account for the majority of sales. Quarterly tacrobel prescriptions (Unit: KRW 100 million, Source: UBIST) This market had been growing steadily until 2022. The tacrolimus immunosuppressant market, which was worth KRW 45 billion in 2020, increased by 7% to KRW 48.4 billion the following year. It then increased by 6% to KRW 51.2 billion in 2022. However, growth accelerated after 2023. In 2023, prescriptions reached KRW 59.3 billion, a 16% increase from the previous year. Last year, the market grew by an additional 10% to KRW 65.2 billion. The market also grew 10% in the first half of this year, continuing the recent high growth trend. Compared to the first half of 2022, before the growth rate accelerated, the market has expanded by 44% from KRW 23.7 billion to KRW 34.9 billion in just three years. Cyclosporine market up 25% in three years... Mycophenolate jumps 67% Another calcineurin inhibitor immunosuppressant, cyclosporine, also showed a similar trend. Cyclosporine has similar indications to tacrolimus and is widely used for suppressing rejection reactions after organ transplants in the liver, kidney, heart, lung, and pancreas, as well as for autoimmune diseases such as psoriasis, rheumatoid arthritis, aplastic anemia, and nephrotic syndrome. Eye drops containing the same ingredient are used to treat conjunctivitis. However, they were excluded from this survey that researched immunosuppressants. This market also saw rapid growth after 2023. Until then, prescriptions had remained in the KRW 30 billion range, posting KRW 28,4 billion in 2020, KRW 30 billion in 2021, and KRW 29.3 billion in 2022. However, it increased by 9% year-on-year to KRW 31.8 billion in 2023, and further expanded by 13% to KRW 35.8 billion last year. In the first half of this year, prescription sales reached KRW 17.7 billion. Compared to KRW 14.2 billion in the first half of 2022, this represents a 25% increase in three years. Quarterly cyclosporine prescriptions (Unit: KRW 100 million, Source: UBIST) Mycophenolate, an anti-metabolite class immunosuppressant, also showed a sharp growth trend around 2023. The mycophenolate market showed fluctuations until 2022, posting KRW 12.7 billion in 2020, KRW 11.7 billion in 2021, and KRW 12.8 billion in 2022. However, in 2023, it increased by 20% year-on-year to KRW 15.4 billion, and last year, it increased by 19% to KRW 18.4 billion. In the first half of this year, it recorded KRW 10.1 billion in prescriptions. Compared to KRW 6 billion in the first half of 2022, it increased by 67% in three years. Quarterly mycophenolate prescriptions (Unit: KRW 100 million, Source: UBIST) Chong Kun Dang leads the immunosuppressant market... The combined prescription sales of the three products reached KRW 28.3 billion in the first half of the year By company, Chong Kun Dang is making a presence in all three immunosuppressant ingredient markets. Chong Kun Dang’s combined prescription sales of Tacrobel, Cipol-N, and My-Rept in the first half of this year reached KRW 28.3 billion, a 5% increase from the same period last year. In the tacrolimus market, Chong Kun Dang’s Tacrobel ranked second after Astellas's Prograf. Tacrobel's prescription sales for the first half of this year reached KRW 8.7 billion, a 7% increase from the KRW 8.2 billion in the same period last year. During the same period, Astellas' Prograf sales increased by 13%, from KRW 16.3 billion to KRW 18.4 billion. In the cyclosporine market, Chong Kun Dang’s Cipol-N dominates the market with over half of the market share. Cipol-N’s prescription sales for the first half of the year were KRW 12.8 billion, maintaining a similar level to last year. Its market share stands at 73%. Novartis' Sandimmune Oral recorded prescription sales of KRW 2.6 billion in the first half of the year. In the mycophenolate market, Chong Kun Dang’s My-Rept also maintained its dominant position with a 67% market share. My-Rept’s first-half prescription sales reached KRW 6.8 billion, a 12% increase from the KRW 6.1 billion recorded in the same period last year. During the same period, Roche's CellCept increased by 15% from KRW 1.6 billion to KRW 1.9 billion, while sales of Novartis' Myfortic rose from KRW 800 million to KRW 1 billion.
- Company
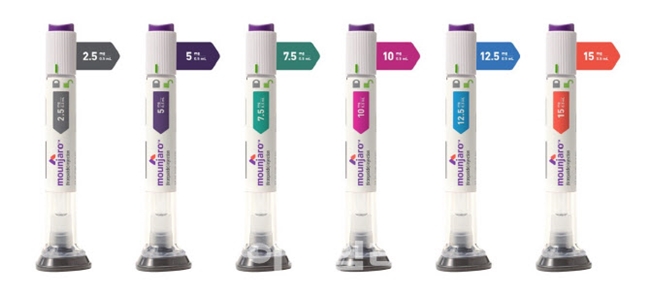
- Mounjaro set for launch in Korea this month
- by Son, Hyung Min Aug 08, 2025 06:02am
- Eli Lilly Korea's diabetes and obesity treatment, Mounjaro, is set to launch in mid-August. Therefore, it will compete against Novo Nordisk's Wegovy. Lilly plans to handle Mounjaro's distribution directly, a strategy that differentiates it from Novo Nordisk, which is currently seeking co-promotion partners. According to the distribution industry on August 8, Lilly Korea plans to launch Mounjaro in Korea this month. The company has organized its own sales and marketing teams and is entering the Korean market through its existing network of distributors. The launch is expected to take place in the third week of August. The domestic supply price for Mounjaro is reportedly around KRW 278,000 for the 2.5mg dose, KRW 369,000 for the 5mg dose, and KRW 521,377 for the 7.5mg and 10mg doses. The initial launch will include only the 2.5mg and 5mg formulations. Mounjaro is a once-weekly subcutaneous injection. Treatment begins with a 2.5mg dose, with the dosage increasing every four weeks. The maximum dose is 15mg. An official from Lilly Korea stated, "We are preparing for the launch of Mounjaro in Korea in the third week of August. We plan to release only the 2.5mg and 5mg doses initially, but we will supply higher-dose formulations, including 7.5mg, 10mg, 12.5mg, and 15mg, without disruption to meet patient demand." Obesity drugs Wegovy, Zepbound, and Saxenda Lilly Korea and Novo Nordisk have adopted different approaches to distributing Mounjaro and Wegovy. Lilly Korea has recently begun contract discussions with over 30 pharmaceutical distributors, including the largest domestic distributor, Ji-O-Young. These negotiations are reportedly in the final stages, with discussions underway with 12 to 15 distributors in the Seoul metropolitan area and 10 to 14 in other regions. For Mounjaro's distribution, Lilly Korea has selected a model in which distributors directly supply healthcare institutions. The company will only be involved in setting the supply price, leaving the actual transaction price to the discretion of the distributors. However, a specific price guide has been established to ensure the product isn't sold "too expensively or too cheaply." Having prior experience in supplying GLP-1 drugs, such as Trulicity, in Korea, Lilly Korea believes it can maintain its competitive edge with this direct distribution model. Novo Nordisk, on the other hand, currently entrusts the nationwide distribution of Wegovy to a foreign-based pharmaceutical distributor, Zuellig Pharma, with supplies also handled by different distributors, such as Bluemtech. Recent reports suggest that Novo Nordisk and domestic pharmaceutical company Chong Kun Dang may enter a co-promotion agreement for Wegovy. Chong Kun Dang has previous experience selling the obesity drug Qsymia from Alvogen Korea. However, according to the company, no final decision has been made. The market is focused on how much Mounjaro will impact Wegovy's dominance. Even with delays in its official domestic launch, Wegovy rapidly captured the market through various channels. According to market research firm IQVIA, Wegovy's sales in the first quarter of this year reached KRW 79.4 billion, accounting for a 73.2% share of the total obesity drug market. Wegovy, a GLP-1 monotherapy containing semaglutide, gained immediate attention following its launch in Korea in October 2024. Despite its high price, demand for prescriptions surged due to its significant weight loss effects. Wegovy quickly rose to the top of the obesity drug market with sales of KRW 60.3 billion in the fourth quarter of last year. The obesity drug market size in the third quarter of last year was KRW 47.4 billion, but it skyrocketed by 97.9% to KRW 93.8 billion in just one quarter with the launch of Wegovy. Mounjaro Demonstrates Superior Weight Loss Effectiveness Over Wegovy Mounjaro is a new diabetes drug developed by Lilly. This treatment, a GIP/GLP-1 receptor dual agonist, works by acting on both Glucose-dependent Insulinotropic Polypeptide (GIP) receptors and Glucagon-like Peptide-1 (GLP-1) receptors. This mechanism promotes insulin secretion, improves insulin resistance, and reduces glucagon secretion, resulting in a decrease in blood sugar levels before and after meal. Major causes of reduced incretin in diabetic and obese patients are decreased GLP-1 secretion and impaired GIP insulin-stimulating effect. GLP-1 and GIP are hormones responsible for two-thirds of the post-meal insulin response. Treatments for diabetes and obesity In Korea, Mounjaro is approved as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes. It is also approved for chronic weight management in obese adults (initial BMI ≥ 30 kg/m²) or overweight adults (initial BMI ≥ 27 kg/m² to < 30 kg/m²) with at least one weight-related comorbidity (e.g., hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease), as an adjunct to a reduced-calorie diet and increased physical activity. Mounjaro has the advantage of not only controlling blood sugar but also demonstrating excellent weight loss effects. Mounjaro proved its weight loss efficacy through the Phase 3 SURMOUNT-1 clinical trial, conducted in obese or overweight adults with at least one comorbidity but without diabetes. Notably, Mounjaro demonstrated superior weight loss effects in SURMOUNT-5, a direct comparative clinical trial against Wegovy. The results of the Phase 3 trial showed that the mean weight loss percentage at 72 weeks for the Mounjaro treatment group (10mg or 15mg) was 20.2%, which was a greater improvement compared to the 13.7% for the Wegovy treatment group (1.7mg or 2.4mg). Having confirmed its weight loss effects in clinical trials for Mounjaro, Lilly launched Zepbound, an obesity treatment with the same active ingredient, in the U.S. market in November 2023. In Korea, both the diabetes and obesity indications will be marketed under the single product name, Mounjaro. Lilly Korea plans to apply for insurance reimbursement for its Mounjaro indication for type 2 diabetes.
- Company
- Donepezil ↑8%, memantine 10%↑…replaces choline alfoscerat
- by Kim, Jin-Gu Aug 07, 2025 06:10am
- Aricept(donepexil), Ebixa(memantine), Sermion(nicergoline) products that are considered alternatives to choline alfoscerate Sales of donepezil, memantine, and nicergoline-based dementia treatments, which have emerged as alternatives to choline alfoscerate formulations, are growing. In the first half of this year, donepezil’s sales recorded KRW 162.6 billion, an 8% increase from the previous year. Sales of memantine-based formulation increased by 10% year-on-year, and nicergoline-based formulations also increased by 36%. Prescription market for the choline alfoscerate alternative ‘donepezil’ grows from KRW 151.1 billion to KRW 162.6 billion in one year According to the pharmaceutical market research institution UBIST on the 6th, the outpatient prescription sales of donepezil-based formulations in the first half of this year amounted to KRW 162.6 billion. This represents an 8% increase from the KRW 151.1 billion in the first half of last year. Donepezil is used to treat symptoms of Alzheimer's dementia. The original product is Hanok's Aricept. In August 2000, Daewoong Pharmaceutical imported the finished product from the original developer and began domestic production and supply. Subsequently, the domestic marketing rights were transferred to Hanok. Sales are handled by Eisai Korea. In 2019, the indication for ‘vascular dementia’ was removed following a clinical reevaluation, but it has had little impact on prescription sales. On the contrary, it has continued to grow by around 5-8% annually since 2020. Prescription sales of donepezil-based formulations increased from KRW 243.6 billion in 2020 to KRW 259.8 billion in 2021, KRW 171.5 billion in 2022, KRW 291.9 billion in 2023, and to KRW 313.9 billion last year. Considering the upward trend in prescriptions in the first half of this year, its sales are expected to continue to increase at the same level as previous years. In particular, sales of generic products have shown marked growth. While the sales of the original product Aricept increased by only 2% from KRW 52.2 billion in the first half of last year to KRW 53.4 billion in the first half of this year, sales of generic products increased by 11% from KRW 98.9 billion to KRW 109.3 billion during the same period. Sales of Daewoong Bio's Beacept increased 14% from KRW 14.9 billion to KRW 17 billion, Samjin Pharmaceutical's Neutoin increased 27% to KRW 6.4 billion in a year, and Whan In Pharm’s Donegil increased 88% to KRW 3.8 billion. The rise of donepezil preparations is analyzed to connected to the crisis of choline alfoscerate products. Choline alfoscerate, which were previously the most widely used in the field of dementia prevention, are facing market withdrawal due to reduced reimbursement for their indications and clinical reevaluations. Initially, choline alfoscerate drugs had three indications: ▲secondary symptoms and degenerative or degenerative brain syndrome caused by cerebrovascular defects; ▲emotional and behavioral changes; ▲senile pseudo-depression. During the clinical reevaluation process, two of the three indications were removed, excluding its use as a treatment of ‘secondary symptoms and degenerative or degenerative brain disorders caused by cerebral vascular defects.’ A separate clinical reevaluation to verify the drug’s safety and efficacy is also underway. If pharmaceutical companies fail to prove efficacy in the clinical reevaluation process, their drugs will have to be completely withdrawn from the market. In addition, they must return 20% of the prescription amounts accrued during the clinical trial period to the health authorities. Given this situation, the pharmaceutical industry has been intent on finding alternatives to replace choline alfoscerate. In this process, donepezil, which has similar indications to choline alfoscerate, has emerged as one of the main alternatives. Although it has been used consistently for dementia prevention, there has been a series of new product approvals since the threat of choline preparations being withdrawn from the market. In fact, since June 2020, when the Ministry of Food and Drug Safety requested companies with choline alfoscerate-based products to submit clinical trial data, 39 pharmaceutical companies have received new approvals for 50 donepezil preparations. Quarterly prescriptions of donepezil products (Unit: KRW 100 million, Source: UBIST) The rise in sales of donepezil-based formulations is analyzed as linked to the crisis of choline alfoscerate drugs. Choline alfoscerate-based formulations, which were previously the most widely used ingredient in the field of dementia p #Sales of memantine-based formulations rise from KRW 27.7 billion to KRW 30.4 billion in one year... Sales of nicergoline preparations also jumped 36% The same situation goes for memantine and nisergoline-based products. These two ingredients are considered major alternatives to donepezil and choline alfoscerate. Prescription of memantine-based products increased 10% in the first half of this year, reaching KRW 30.4 billion, compared to the KRW 27,7 billion in the same period last year. Memantine is indicated for the treatment of moderate-to-severe Alzheimer's disease. The original product is Lundbeck's ‘Ebixa,’ which was approved in 2003. Like donepezil-based formulations, new product approvals have skyrocketed since June 2020. Twenty-seven pharmaceutical companies have been approved for 37 products in the last five years. One in three of all memantine products (109) on the market has been approved since the controversy arose over the efficacy of choline preparations. At the end of last year, approvals for new combination products that contain memantine and donepezil followed one after another. After HyundaiPharm received approval for ‘DM Duo,’ eight companies obtained approval for 14 additional products. Related products collectively achieved prescription sales of KRW 500 million in the first half of this year. Among choline esterase inhibitor alternatives, nicergoline-based formulations have had the most new product approvals in the industry. Nicergoline is approved for the ‘primary treatment of dementia symptoms such as memory impairment, concentration disorders, judgment disorders, and lack of initiative associated with primary degenerative vascular dementia and mixed dementia.’ The original product is Ildong Pharmaceutical’s Sermion. Ildong Pharmaceutical received approval for this product in 1997. Until 2022, no subsequent products were approved, excluding those for export. However, since 2023, new product approvals have been granted one after another. Following Hanmi Pharmaceutical's approval of ‘Nicegoline’ in January 2023, 39 companies have received approval for 53 products as of recently. Prescription sales of nicergoline formulations increased by 36% from KRW 3.3 billion in the first half of last year to KRW 4.5 billion in the first half of this year. In particular, the growth of new follow-on products that were recently released to the market has been rapid. The combined prescription sales of the follow on products, which amounted to only KRW 400 million in the first half of last year, increased more than threefold to KRW 1.5 billion in just one year. Sermion’s sales also increased slightly from KRW 2.9 billion to KRW 3 billion. Sales of existing choline preparations recorded KRW 294 billion in the first half of this year. Although this represents a slight decrease from the KRW 301.4 billion in the first half of last year, it still boasts prescription sales of nearly KRW 300 billion, demonstrating its continued popularity. Daewoong Bio and Chong Kun Dang, which hold the top two positions in the existing choline alfoscerate market, are also actively seeking alternatives. Daewoong Bio's ‘Gliatamin’ and Chong Kun Dang's ‘Chongkundang Gliatirin’ accounted for more than half of the total choline preparation market in the first half of the year. In preparation for the withdrawal of its choline alofscerate products, Daewoong Bio has received approval for ‘Beacept,’ which contains donepezil, ‘Glibixa’ containing memantine, and ‘Daewoong Nicergoline’ containing nicergoline. Chong Kun Dang has obtained approval for ‘Neuromanthyn’ containing memantine and ‘Nexcholine’ containing nicergoline. Additionally, at the end of last year, it added ‘Neurocept Duo,’ a combination of donepezil and memantine.
- Company
- Yuhan’s Leclaza to receive ₩100B in milestone payment
- by Chon, Seung-Hyun Aug 07, 2025 06:06am
- With Yuhan Corp’s new anticancer drug Leclaza approved in China, the company announced that it will receive KRW 63 billion in technology fees. Added to the technology fees secured at the end of last year for the drug’s approval in Europe, over KRW 100 billion in additional milestone payments are expected to be paid to the company. As a result, Yuhan Corp has secured a total of over KRW 400 billion in technology fees and sales royalties for Leclaza. 1 According to industry sources, the Chinese National Medical Products Administration (NMPA) recently included Leclaza in its list of newly approved drugs. Leclaza has been approved as a first-line treatment for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation in combination with Johnson & Johnson's Rybrevant. The drug is a non-small cell lung cancer treatment that was approved as the 31st domestically developed new drug in January 2021. Yuhan Corp exported the technology for Leclaza to Janssen Biotech in November 2018. Yuhan Corp will receive an additional $45 million in milestone payments from Janssen when sales of Leclaza begin in China. The combination therapy of Leclaza and Rybrevant was approved by the US Food and Drug Administration (FDA) in August last year and received approval from the European Commission (EC) at the end of last year. Japan's Ministry of Health, Labour and Welfare approved the combination therapy in March. Yuhan Corporation has not yet received the USD 30 million milestone payment for Leclaza's European approval. The fee will be paid once Leclaza goes on sale in major European countries. This means that Yuhan has secured a total of USD 75 million (approximately KRW 100 billion) in technology fees from China and Europe. Yuhan Corp has received a total of USD 225 million from Janssen since signing the licensing out deal for Leclaza. Yuhan Corp received USD 50 million in November 2018 as an upfront payment for Leclaza’s licensing out deal. Yuhan Corporation then received a milestone payment of USD 35 million from Janssen in April 2020. At that time, Johnson & Johnson paid Yuhan an additional milestone payment as it began clinical trials of Rybrevant in combination with Leclaza. Johnson & Johnson paid Yuhan an additional milestone payment of USD 65 million in November 2020 as it began recruiting subjects for clinical trials. Then, the company received an additional USD 60 million in milestone payments last year with the approval of Leclaza in the United States. In May, it received an additional KRW 15 million with the launch of Leclaza in Japan. Yuhan Corporation posted KRW 25.5 billion in milestone payments in the second quarter from the Japanese approval. With the addition of payments to come from China and Europe, Leclaza's technology fees will total near USD 300 million (KRW 420 billion). Yuhan Corporation receives a percentage of Leclaza's overseas sales as sales royalties. In other words, the more Leclaza's global sales expand, the more its revenue expands. According to Johnson & Johnson, the combination therapy of Leclaza and Rybrevant recorded sales of USD 320 million in the first half of this year. Forty percent of the technology fee revenue secured by Yuhan Corp will be paid to Oscotec, the original developer. Yuhan Corporation acquired the rights to develop Leclaza, which was in the preclinical stage, from Oscotec and its subsidiary Genosco in 2016. The total contract value was worth KRW 1.5 billion. Oscotec received KRW 1.289 billion in sales royalties from Leclaza in the first quarter of this year.
- Company
- 'Brintellix', stable position in antidepressant drugs mkt
- by Eo, Yun-Ho Aug 07, 2025 06:05am
- Product photo of Brintellix 'Brintellix' has established a stable presence in the antidepressant drugs market over the past 10 years. Its presence has not been disturbed by issues such as generic entries. According to industry sources, Lundbeck Korea's Brintellix (vortioxetine) has been growing in the market for antidepressant drugs for the past 10 years. In Q1 20125, Brintellix ranked No.2 in sales in the antidepressant market, based on the IQVIA data. Brintellix, first introduced in Korea in 2015, is differentiated from existing antidepressants, such as Selective Serotonin Reuptake Inhibitors (SSRIs) or Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), which work by blocking the reuptake of serotonin or norepinephrine to produce an antidepressant effect. Brintellix is a new antidepressant with a mechanism that acts on various serotonin receptors while also inhibiting serotonin reuptake. Through this, it not only balances various neurotransmitters in addition to serotonin but also shows an effect in improving depressive symptoms and cognitive symptoms (such as concentration, attention, learning ability, and executive function) in patients with Major Depressive Disorder (MDD). Brintellix has been proven to significantly improve cognitive symptoms such as reduced attention, lack of concentration, and memory impairment that are observed in MDD patients. Furthermore, this drug was confirmed to significantly improve symptoms of reduced motivation and lack of energy in depressed patients, and it also statistically and meaningfully improved emotional blunting. These improvements in cognitive and emotional symptoms were not limited to short-term treatment. Brintellix demonstrated sustained antidepressant effects through a long-term study lasting 52 weeks and was reported to reduce the relapse rate in MDD patients by approximately 50% compared to placebo. Additionally, efforts to enhance the administration convenience for Brintellix are underway. Lundbeck is currently conducting clinical trials to compare the safety and pharmacokinetic properties of a salt-changed product of Brintellix upon administration. An official of Lundbeck stated, "As we celebrate the 10th year of Brintellix launch in Korea, Lundbeck has a variety of plans to help more patients based on its years of clinical experience and reliable products. We plan to contribute to treating depression with a patient-centered treatment option."
- Company
- COVID-19 vaccines transitioned to NIP
- by Whang, byung-woo Aug 06, 2025 06:10am
- COVID-19 vaccines, which were previously contracted through a pre-purchase model with pharmaceutical companies, will transition to the National Immunization Program (NIP) system. The Korea Disease Control and Prevention Agency (KDCA) announced on August 5 that it has signed a procurement contract for the supply of vaccines for the 2025-2026 seasonal COVID-19 immunization program. Unlike the previous method (2020-2024), where contracts were signed through a pre-purchase model with pharmaceutical companies using full government funding, this COVID-19 vaccine contract was signed through a government procurement model, transitioning it to a local government-subsidyzed project (matching local government funds), which is the same as the existing NIP system. The COVID-19 vaccines to be supplied to Korea for the 2025-2026 season are vaccines with the LP.8.1 strain, which have been recommended for use by the WHO (World Health Organization), EMA (European Medicines Agency) on May 16, and FDA (U.S. Food and Drug Administration). The decision was made by the Korea Expert Committee on Immunization Practices (KECIP). The total vaccine volume to be procured is 5.3 million doses (3.28 million from Pfizer and 2.02 million from Moderna), and contracts were signed through their respective COVID-19 vaccine distributors (companies with exclusive sales rights) in Korea. The domestic distributor for the Pfizer vaccine is HK inno.N, and Boryung Biopharma is the domestic distributor for Moderna. Regarding this contract, the KDCA explained, "The COVID-19 vaccine supply contract in Korea was signed using a private contract method, rather than a competitive bid, to ensure stable supply. However, based on a survey of local government demand, we incorporated price competitiveness elements and an additional reserve volume (5%) for each pharmaceutical company, aiming for both stable vaccine supply and budget savings." To minimize vaccine waste, vaccines nearing their expiration date during the project period can be exchanged to ensure continuous use throughout the vaccination period. After the project concludes, remaining vaccines can be returned within a 5% limit of the contracted volume. To minimize vaccine waste, vaccines nearing their expiration date during the project period can be exchanged to ensure continuous use throughout the vaccination period. After the project concludes, remaining vaccines can be returned within a 5% limit of the contracted volume. Meanwhile, Moderna announced that it will strive to protect high-risk groups aged 65 and over in Korea through the 2025-2026 seasonal NIP. Moderna's COVID-19 vaccine has accumulated extensive real-world vaccination data in Korea, with approximately 29.18 million doses administered from the beginning of the pandemic until now, confirming its efficacy and safety. According to a study conducted by the KDCA, the Moderna vaccine was found to have the lowest breakthrough infection rate among the vaccines used in Korea during the early stages of the pandemic. Since 2021, Moderna has collaborated with Samsung Biologics to produce the only mRNA COVID-19 vaccine in Korea. In addition, Moderna is making efforts to strengthen mRNA technology capabilities through various collaborations, including joint research on a vaccine candidate for Severe Fever with Thrombocytopenia Syndrome (SFTS) with the National Institute of Infectious Diseases under the KDCA, and a partnership with KAIST and Yonsei University K-NIBRT for training mRNA research talent. Kim Sang-pyo, General Manager of Moderna Korea, said, "We are deeply grateful and find it meaningful that Moderna's efforts over the past few years to protect high-risk groups from the COVID-19 can continue through this year's NIP," and added, "As COVID-19 remains an infectious disease with a high severity rate, especially in adults aged 65 and over, we will put utmost efforts to protect more high-risk individuals in line with the government's vaccination plan."
- Company
- Imfinzi wins nod as pre- and post-operative adjuvant therapy
- by Whang, byung-woo Aug 06, 2025 06:10am
- Product photo of Imfinzi AstraZeneca announced on August 4 that it has received approval for its Imfinzi (durvalumab) as pre- and post-operative adjuvant therapy for patients with muscle-invasive bladder cancer from the Ministry of Food and Drug Safety on July 30. With this approval, Imfinzi has become the first and only immunotherapy in Korea to be approved for pre- and post-operative adjuvant therapy in the treatment of muscle-invasive bladder cancer. Imfinzi's specific approved indication is for 'the treatment of patients with muscle-invasive bladder cancer as a neoadjuvant combination therapy with cisplatin and gemcitabine or as Imfinzi monotherapy as adjuvant therapy after radical cystectomy.' Imfinzi's specific approved indication is for 'the treatment of patients with muscle-invasive bladder cancer as a neoadjuvant combination therapy with cisplatin and gemcitabine or as Imfinzi monotherapy as adjuvant therapy after radical cystectomy.' The previous standard therapy was neoadjuvant chemotherapy followed by radical cystectomy. However, this left an unmet need due to high recurrence rates and poor prognosis even after treatment. It is known that approximately 50% of muscle-invasive bladder cancer patients experience recurrence within three years. The expanded indication is based on the Phase 3 NIAGARA clinical study, which evaluated the clinical efficacy and safety of Imfinzi as a pre- and post-operative adjuvant therapy for muscle-invasive bladder cancer. In this study, patients were divided into two groups. The experimental group received Imfinzi and chemotherapy (gemcitabine-cisplatin) in combination as neoadjuvant therapy for 4 cycles at 3-week intervals, followed by surgery and then Imfinzi monotherapy as adjuvant therapy for 8 cycles at 4-week intervals. The control group underwent chemotherapy (gemcitabine-cisplatin) as neoadjuvant therapy, followed by surgery only. The study results showed that Imfinzi's pre- and post-operative adjuvant therapy significantly improved one of the key primary endpoints, event-free survival (EFS), compared to the control group. The 2-year event-free survival rate was 67.8% for the Imfinzi pre- and post-operative adjuvant therapy group and 59.8% for the control group. The Imfinzi pre- and post-operative adjuvant therapy reduced the risk of disease progression, recurrence, failure to undergo radical cystectomy (surgery), and death from all causes by 32%. In terms of the safety profile, Imfinzi's results were consistent with the individual safety profiles of previously confirmed Imfinzi and chemotherapy (gemcitabine-cisplatin). Based on these clinical benefits, Imfinzi is a Category 1 preferred recommendation for pre- and post-operative adjuvant therapy for patients with muscle-invasive bladder cancer in the National Comprehensive Cancer Network (NCCN) guidelines. Furthermore, the European Society for Medical Oncology (ESMO) has rated it with an A-grade, the highest rating in their Magnitude of Clinical Benefit Scale (MCBS) framework. Professor Byong Chang Jeong of the Department of Urology at Samsung Medical Center (President of the Korean Urological Oncology Society) explained, "The previous standard therapy for muscle-invasive bladder cancer had limitations in improving patient survival due to high recurrence rates," and added, "The newest approval of an immunotherapy as a pre- and post-operative adjuvant therapy is a favorable change in the clinical field." Jeong added, "By administering Imfinzi, we can expect to increase the success rate of surgery through neoadjuvant therapy and reduce the recurrence rate through adjuvant therapy, ultimately improving the survival rate of patients with muscle-invasive bladder cancer in Korea."