- LOGIN
- MemberShip
- 2025-12-20 23:09:05
- Policy
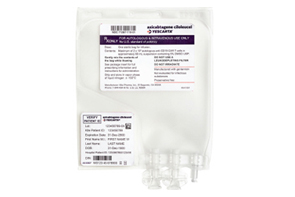
- Gilead’s Yescarta approved in KOR...3rd CAR-T therapy
- by Lee, Hye-Kyung Aug 14, 2025 06:13am
- The third CAR-T therapy has been approved in Korea. The Ministry of Food and Drug Safety approved Gilead Sciences Korea's Yescarta (axicabtagene ciloleucel) today (13th). Yescarta is a CAR-T therapy that received approval from the U.S. FDA in 2017 and the European EMA in 2018. Last year, the EMA approved it as a second-line treatment for patients with adult diffuse large B-cell lymphoma and B-cell acute lymphoblastic leukemia. In June, the FDA expanded the indication for Yescarta to include patients with follicular lymphoma. In Korea, after the drug was designated as an orphan drug by the MFDS in September last year, it has been undergoing the formal approval process. According to a brief issued by the Korea Biotechnology Organization, Yescarta is currently the CAR-T therapy with the highest market share worldwide. Posting sales of USD 1.5 billion (approximately KRW 2 trillion) last year, Yescarta ranked first with a 40% share of the entire CAR-T market. It was followed by Novartis' Kymriah posting USD 500 million (approximately KRW 700 billion), Johnson & Johnson's ‘Carvykti’ at USD 500 million, BMS's ‘Abecma’ at USD 470 million (approximately KRW 650 billion), and Gilead Sciences' ‘Tecartus’ at USD 370 million (approximately KRW 500 billion). Kymriah and Carvykti have already been granted marketing authorization in Korea. Meanwhile, according to an analysis presented at the American Society of Clinical Oncology (ASCO) 2023 Annual Meeting, at a median follow-up of 47.2 months, the overall survival (OS) in the Yescarta treatment group had not yet reached the median, while the placebo group had a median OS of 31.1 months, indicating a statistically significant 27% lower risk of death with Yescarta. The estimated 48-month overall survival rate was 54.6% for Yescarta and 46.0% for the control group, with Yescarta demonstrating consistent survival benefits across pre-specified subgroups, including age, primary resistance, early relapse, and high-grade B-cell lymphoma.
- Company
- 'Tevimbra' applies for reimbursement for five indications
- by Eo, Yun-Ho Aug 13, 2025 06:07am
- Product photo of Tevimbra 'Tevimbra' is quickly expanding following its successful inclusion in the insurance reimbursement for esophageal cancer. BeOneMedicines Korea has been confirmed to have applied for reimbursement on the day of approval for five new indications for its PD-1 inhibitor immunotherapy Tevimbra (tislelizumab), which received additional approval in June. In April, Tevimbra became the first immunotherapy to receive reimbursement for esophageal cancer. Following this, it has added five new indications for solid tumors, including esophageal cancer, gastric cancer, and non-small cell lung cancer. The specific expanded indications are ▲first-line combination therapy for unresectable, locally advanced, or metastatic esophageal cancer ▲first-line combination therapy for unresectable or metastatic, HER2-negative gastric or gastroesophageal junction adenocarcinoma ▲two types of first-line combination therapy, and second-line monotherapy for non-small cell lung cancer. Tevimbra has already received approval from the U.S. FDA for first- and second-line treatment of unresectable or metastatic esophageal squamous cell carcinoma, as well as for first-line treatment of advanced gastric cancer. The European Medicines Agency (EMA) has also approved it for first- and second-line treatment of esophageal squamous cell carcinoma, first-line treatment of advanced gastric cancer, and first- and second-line treatment of non-small cell lung cancer. Tevimbra is receiving various expanded indication in the global market. Since the company, BeOne Medicines, has been quick to proceed with reimbursement procedures for additional indications, Tevimbra's role is expected to expand across various cancer types in Korea. Expectations are particularly high due to BeOne Medicines' previous track record of successfully concluding negotiations with the government by advocating for 'reasonable drug pricing' since its initial listing. The reason for the low reimbursement rate by indication for immunotherapies, including esophageal cancer, is, as expected, related to drug pricing and financial burden. After reimbursement was applied to some cancer types, like lung cancer, the total amount claimed for immunotherapies and their share of the total cancer drug claims within the health insurance system significantly increased, raising concerns about the financial burden. As of 2023, cancer drug claims totaled KRW 2.4 trillion, with immunotherapy claims accounting for approximately KRW 500 billion, or 20% of the total cancer drug claims. It remains to be seen whether BeOne Medicines can uphold its company philosophy of 'providing innovative new drugs at a reasonable price and leaving no patient behind.' Meanwhile, the efficacy and safety of Tevimbra were proven in various indications through the RATIONALE clinical trial series (RATIONALE-303, 304, 305, 306, 307). In esophageal squamous cell carcinoma and gastric or gastroesophageal junction adenocarcinoma, Tevimbra demonstrated clinical benefits in the overall patient population. The trials showed consistent results in pre-specified subgroups based on PD-L1 expression.
- Policy
- New drug approval fees ₩410mil in KOR
- by Lee, Hye-Kyung Aug 13, 2025 06:07am
- Since the new drug approval fee was significantly increased to KRW 410 million starting this year, a total of 14 new products containing 10 ingredients have been submitted for approval. Although the specific product names cannot be disclosed, dedicated teams have been formed for 6 chemical drug substances and four biopharmaceutical substances, and the review process is currently underway. On the 12th, Young-joo Kim, Director of the Drug Approval Division at the Ministry of Food and Drug Safety, So-hee Kim, Director of the Director of the Cardiovascular Drug Division of the Drug Review Department, and Jae-ok Kim, Director of the Biological Products Division of the Bio and Herbal Medicine Review Department, met with specialized media journalists to provide an interim briefing on the new drug approval and review innovation process that began this year. , Young-joo Kim, Director of the Drug Approval Division at the Ministry of Food and Drug Safety, So-hee Kim, Director of the Director of the Cardiovascular Drug Division of the Drug Review Department, and Jae-ok Kim, Director of the Biological Products Division of the Bio and Herbal Medicine Review Department On January 1, the MFDS raised the new drug approval fee to KRW 41 million, formed dedicated teams for each item, expanded face-to-face consultations and reviews between companies and approval reviewers for items submitted for approval to a maximum of 10 times, and shortened the manufacturing and quality control evaluation and actual condition survey of new drug manufacturing facilities (to within 90 days). The increased approval fees were to be used mostly to hire high competency reviewers, and the MFDS has established a policy to operate a professional, swift, transparent, and predictable approval review system so that the process from new drug approval application to license issuance can be completed within 295 days. The new drug approval and review process begins with a pre-submission consultation, followed by the submission of a product license application. A dedicated team of approximately 15 members is then formed for each product. Actual meetings begin within 2 weeks of the submission of the application. GMP inspections for new drugs are also completed within 90 days of the submission date. On January 31, multinational pharmaceutical company Eli Lilly applied for marketing authorization for its new breast cancer drug “Inluriyo Tab (imlunestrant),” and a total of 10 substances are currently under review. While actual marketing authorizations are not completed in the order of application submission, for Inluriyo, if the innovative approval scheme for new drugs is applied, approval could be granted as early as November or by December at the latest. Director Young-Joo Kim stated, “While I cannot comment on the approval process for specific products, we are striving to complete the approval process within 295 days from the date of submission of the marketing authorization application in accordance with the new drug approval and review procedures. Despite the increase in new drug approval fees, we anticipate that the number of marketing authorization applications submitted will be similar to previous years.” As of August, applications for new drug approvals for 10 ingredients have been submitted, and based on the usual number of applications, applications for 20 ingredients were submitted in 2023 and 19 ingredients in 2024. The MFDS believes that if this trend continues, the number of item approval applications will remain similar to those of previous years. Kim added, “Until last year, when requests for supplementary data were made, everything was handled via documents. However, now, there are over 10 face-to-face meetings from preliminary consultations to supplementary data submission requests, and all of this process is documented.” However, despite the significant increase in fees, only 15 to 17 dedicated staff members are being assigned to expedite approvals. This is why, when a meeting is held regarding supplementary materials, everyone has to gather for a meeting lasting over an hour and a half. Director Jae-ok Kim explained, “One hour and a half may seem short, but when supplementary materials are submitted, pharmaceutical companies also select the points they want to focus on and ask questions. The meeting lasts about an hour and a half, with the purpose of the supplementary data request being explained and any questions being answered.” In the case of new drug approval fees, most of the increased amount will be used to cover the pay of high competency reviewers. Currently, there are 31 high competency reviewers hired specifically for the new drug team, which is close to the total quota of 36. The new drug dedicated team is composed of a team leader who is the head of the approval department, a product manager from the approval department, and specialists in safety and efficacy, quality control, GMP (good manufacturing practice), and GCP (good clinical practice), rendering the need to reinforce the specialized review staff. Director Young-joo Kim said, “With the increase in new drug approval fees, the cost of review is borne by the beneficiaries. The MFDS has a total of 370 reviewers, including those for medical devices, and about 10% of them are high competency reviewers.” However, as high competency reviewers are hired as public employees rather than civil servants, it is difficult for them to provide stable service. As a result, they may only receive training on high competency review techniques and move to other organizations within a few years. Director Jae-ok Kim added, “The current review staff is going through a transitional period. High competency reviewers cannot be immediately deployed; they must first undergo training, work as assistant reviewers, and then transition to head reviewers." He also pointed out that “this process is causing an overload of work for existing review staff.” Director So Hee Kim also emphasized, “Since the system is still in its initial stages, mid-level managers are facing significant challenges. While hiring high-skilled reviewers is important, expanding the pool of personnel capable of fulfilling mid-level management roles is also necessary.” In this regard, Director Young-joo Kim said, “It is important to enable high competency reviewers hired as public officials to be appointed civil servants,” adding, “We plan to consult with other ministries and request that the number of civil servants be continuously increased.” Although the MFDS is applying the new drug approval fee hike only to new drugs, it plans to gradually expand it to other items. President Jae-myung Lee's remarks also support this stance. At a recent cabinet meeting, the president stated, “Insufficient review costs and a shortage of personnel are causing delays in new drug approvals, which is a loss for society as a whole. Increasing review costs is a way to expand the budget without increasing the financial burden.” Director Young Joo Kim said, “The MFDS understands that the intention is not to further raise the new drug approval fees, but to expand the fees to items other than new drugs to increase revenue. We are in ongoing discussions with government ministries and related industries on items for which fees can be raised based on principles such as the novelty and complexity of the substance.”
- Product
- Mounjaro set to hit shelves next week... pricing a dilemma
- by Jung, Heung-Jun Aug 13, 2025 06:07am
- With the imminent arrival of the diabetes and obesity treatment Mounjaro (tirzepatide) in Korea, pharmacies are struggling to set an appropriate price for the item. Due to the nature of high-priced non-reimbursable drugs, there are regional price differences, and price competition is expected in the future, so pharmacies are showing a cautious stance on their initial sales price. Recently, some distributors notified pharmacies of Mounjaro ‘s supply schedule for the 20th and surveyed the pharmacies’ willingness to handle the product to anticipate demand. Additionally, telemedicine platforms are guiding affiliated clinics and pharmacies to input their planned selling prices. Like Wegovy, Mounjaro’s supply price was disclosed before its domestic launch. However, unlike Wegovy, the supply price varies by dosage. While there may be minor differences depending on the distributor, the prices are currently set at KRW 278,000 for 2.5mg and KRW 369,300 for 5mg. The prices for the upcoming 7.5mg and 10mg doses are reported to be KRW 521,300. Pharmacists are expected to monitor the prices set by nearby clinics and pharmacies before determining their own sales prices. Pharmacist A from Seoul stated, “Based on the 2.5mg dosage, the price is expected to range from the mid-300,000 KRW to the 400,000 KRW bracket. Although distribution is scheduled for next week, we have not yet decided whether to carry the product, as we are unsure whether hospitals will issue outpatient prescriptions.” Pharmacist A added, “Although both products are used for obesity treatment, their mechanisms of action are different, so it is not appropriate to simply compare their dosage and price.” In the case of Wegovy, the selling price, which was in the range of KRW 500,000 to KRW 600,000 when it was first launched in Korea, gradually decreased, and the price was adjusted. In the case of obesity treatments, price information is quickly shared through telemedicine platforms and communities, so even among non-reimbursable drugs, the sales price can fluctuate greatly. Some pharmacists are hesitant to handle the drug because the supply price is known and there is a risk that it could become dead stock. Pharmacist B from Seoul stated, “It has a competitor, so it's difficult to predict how much demand there will be for Mounjaro, so we're waiting to see how demand develops. We also have remaining stock of Saxenda and Wegovy. Price competition is intense, so expanding our inventory can be a burden.”
- Company
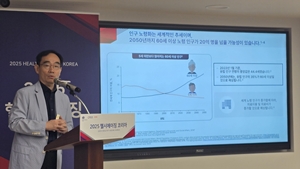
- Adult vaccination in a super-aged society highlighted
- by Whang, byung-woo Aug 13, 2025 06:07am
- South Korea has entered a super-aged society, with its population aged 65 and over exceeding 20%. The need for a response through adult vaccination is being emphasized. With the number of National Immunization Program (NIP) vaccines for adults limited to just influenza and pneumococcal vaccines, there is a growing opinion that the program needs to be expanded. The British Embassy in Seoul, the British Chamber of Commerce in Korea, and GSK Korea jointly hosted the '2025 Healthy Ageing Korea' forum on August 12 to discuss the necessity of adult vaccination. Professor Kwang-il Kim of the Department of Geriatrics at Seoul National University Bundang Hospital Professor Kwang-il Kim of the Department of Geriatrics at Seoul National University Bundang Hospital gave a presentation on 'Adult Vaccination for an Extended Healthy Lifespan'. Professor Kim emphasized the need for a full-lifecycle vaccination program as a healthcare policy to prevent infections and extend healthy lifespans in an aging society. Professor Kim stated, "While the consumption of medical resources and the social burden continue to increase among the elderly population in Korea, there are not many vaccines included in the National Essential Vaccination Program for adults, many of whom have chronic diseases that make them vulnerable to infectious diseases." In fact, NIP vaccines for adults are limited to two types: influenza and pneumococcal. Other adult vaccines are supported through small-scale vaccination projects using local governments' own budgets, leading to issues of fairness between regions. Professor Kim said, "Vaccination in the elderly can contribute to healthy aging by preventing disease-related complications, thereby reducing the medical burden and mortality rates." He added, "Diverse policy and strategic approaches to vaccination are needed for an aging society and healthy aging." Professor Hankil Lee of Ewha Womans University's College of Pharmacy gave a presentation on 'The Value of Adult Vaccination in Response to a Super-Aged Society', highlighting adult vaccination as a crucial public health policy for an aging population. According to Professor Lee, as the effectiveness of vaccination has been proven in the elderly, major overseas countries are including adult vaccination in their NIPs and supporting it with public funds. The UK offers free shingles vaccination for its elderly population, and Japan has been supporting shingles vaccines with a mixed financial structure since April of this year. Professor Hankil Lee of Ewha Womans UniversityProfessor Lee's opinion is that adult vaccination not only protects individuals from infectious diseases but also contributes to improving public health and reducing the socioeconomic burden at a national level, thereby generating economic effects from various perspectives. Professor Lee said, "In Korea, adult vaccination has not been fully recognized as a public good with sufficient social benefits from the perspective of responding to a super-aged society." He emphasized, "The policy gap in adult vaccination continues to exist, and it must be systematically addressed when establishing a comprehensive national vaccination plan in the future." The forum also revealed the results of a cost-benefit analysis of shingles and RSV (Respiratory Syncytial Virus) vaccines in adults in Korea. The analysis showed that for shingles vaccines in individuals aged 50 and over, the Return on Investment (ROI), or socioeconomic benefit relative to cost, was approximately 1.52. For RSV vaccines in the elderly aged 60 and over, the socioeconomic benefit was 1.65. Professor Lee said, "When the socioeconomic benefit exceeds 1, it is considered that a larger social benefit was generated than the cost incurred. This analysis proves that adult vaccination is a public investment that brings long-term socioeconomic benefits, going beyond just disease prevention." Colin Crooks, British Ambassador to the Republic of Korea, added, "While the elderly population is increasing globally, Korea is aging at a particularly fast pace." He continued, "In this situation, this forum was a meaningful opportunity for academia, the government, and the elderly community to discuss the protection of the health rights of the elderly and the necessity of prevention. It is expected to be an opportunity to raise awareness of the importance of prevention-centered public health across society."
- Product
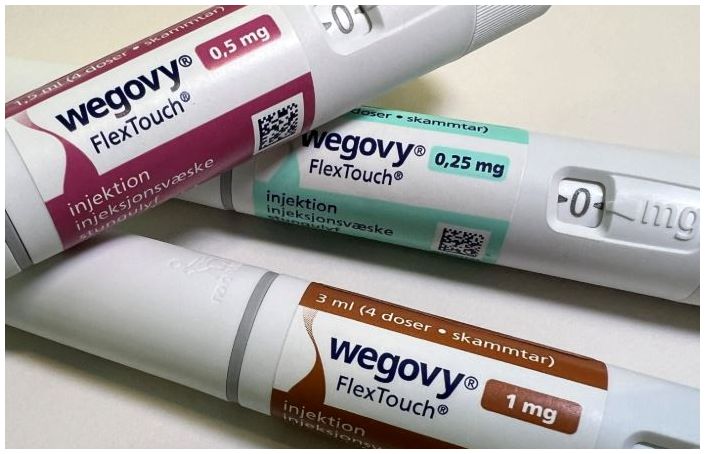
- Wegovy supply price cut by 40%... to counter Mounjaro
- by Kang, Hye-Kyung Aug 13, 2025 06:06am
- The supply price for Wegovy will be reduced by approximately 40% starting on the 14th. Refund measures are also expected to be implemented for pharmacies with inventory. Novo Nordisk notified pharmacies and other relevant parties of the changes through Julic and other channels. According to Dailypharm's coverage, the prices for four dosage forms—0.25mg, 0.5mg, 1.0mg, and 1.7mg—will be reduced, excluding the highest dosage of 2.4mg. The previous single price system will be replaced with a tiered pricing system based on dosage, with the 0.25mg starting dosage set to receive a price cut of around 40%. Once the price reductions are implemented, the purchase prices for pharmacies and clinics will be around KRW 220,000. However, to prevent confusion in medical and pharmacy settings, the specific price reduction rates will not be disclosed. Refunds for existing inventory held by pharmacies will also be processed according to established procedures. Details regarding refund procedures and amounts per pharmacy will be communicated sequentially through Zuellig Pharma sales representatives and the suppliers. Some have speculated that the price reduction policy for Wegovy may be in response to the upcoming launch of Mounjaro this month. Mounjaro, which is expected to be launched around mid-month, will apply tiered pricing across six dosage forms, with the starting dosage being approximately KRW 100,000 cheaper than Wegovy. However, Novo Nordisk stated that it had been reviewing a tiered pricing policy by dosage for a long time after the launch and had decided to switch from a uniform single price system to a tiered pricing system by dosage. A Novo Nordisk representative said, “We decided to lower the shipping price and notify customers to minimize confusion among distributors, pharmacies, and patients who have been prescribed the drug.” They added, “In supplying innovative treatments like Wegovy, we prioritize treatment continuity and accessibility for Korean obesity patients as our top principle, and this principle also applies when determining the shipping price of the treatment. We will continue to strive to improve the obesity treatment environment in Korea through a patient-focused approach.”
- Company
- Tariff impact inevitable for biodrugs’ export to the US
- by Cha, Jihyun Aug 12, 2025 06:16am
- Exports of domestically produced biopharmaceuticals to the US more than doubled last year compared to the previous year. If the Trump administration's tariffs on pharmaceuticals are implemented, the domestic biopharmaceutical export industry is expected to suffer significant damage. According to the “Key Data on the Domestic Biopharmaceutical Industry 2025” published by the Korea Biomedicine Industry Association (KoBIA, President: Jeong-seok Lee) on the 11th, last year's exports of domestically produced biopharmaceuticals to the United States reached USD 686.7 million (approximately KRW 845.9 billion). This represents a 103% increase from the previous year's USD 300.72 million (approximately KRW 417.8 billion). Last year, the export value of domestically produced biopharmaceuticals to the U.S. grew to nearly match the import value. Last year, the U.S. imported 694.7 million dollars worth of biopharmaceuticals, a 7% increase from the previous year. The share of North America in the total exports of domestically produced biopharmaceuticals also expanded significantly over the past year. By continent, the North American region's share increased by 5 percentage points, from 14.7% in 2023 to 19.4% last year. The US ranked second in biopharmaceutical exports, following Hungary. Hungary's biopharmaceutical exports totaled USD 1.23346 billion, followed by Turkey (USD 432.06 million) and Brazil (USD 144.04 million). The analysis is that exports of biopharmaceuticals to the United States have been increasing in line with the growth of the domestic biosimilar industry. Biosimilars developed by domestic companies such as Celltrion and Samsung Bioepis are rapidly expanding their market share in the United States. Celltrion received four biosimilar approvals in the US this year alone, while Samsung Bioepis launched “Pyzchiva,” a biosimilar version of the autoimmune disease treatment “Stelara,” and ‘Epysqli,” a biosimilar version of the rare disease treatment “Soliris” in the US market this year. (Source:KoBIA) Top 10 biopharmaceutical export/import nations With exports of biopharmaceuticals to the U.S. on the rise, domestic companies are closely monitoring the Trump administration's tariff imposition. As the U.S. is a major export destination for Korean biopharmaceuticals, there are concerns that the imposition of tariffs will inevitably deal a blow to the domestic biopharmaceutical export industry. U.S. President Donald Trump recently announced plans to raise tariffs on certain pharmaceutical products by up to 250%. President Trump stated, “We will impose tariffs on certain pharmaceutical products soon, raise the tariff rate to 150% after one year, and then to 250% thereafter.” As the U.S. is set to announce the results of its Section 232 investigation under the Trade Expansion Act of 1962, the industry believes that the imposition of tariffs on pharmaceutical products is highly likely. Section 232 of the Trade Expansion Act allows the Department of Commerce to investigate the impact of imported goods on national security and enables the president to take appropriate measures. The US government launched an investigation into this matter in April. In line with this policy direction, domestic and foreign pharmaceutical and biotechnology companies have been accelerating their production investments in the US. AstraZeneca and Roche have each announced investments of USD 50 billion, Johnson & Johnson USD 55 billion, Eli Lilly USD 27 billion, Novartis USD 23 billion, and Sanofi at least USD 20 billion to expand their manufacturing capabilities in the US. Domestic companies such as Celltrion are also rushing to enter the U.S. market and secure production bases. Celltrion is reportedly in talks to acquire Eli Lilly's monoclonal antibody production plant in Branchburg, New Jersey. Earlier, Jung Jin Seo, chairman of Celltrion Group, stated that the company is pursuing the acquisition of a U.S. active pharmaceutical ingredient (API) plant to reduce uncertainties related to U.S. tariff risks.
- Company
- 'Vanrafia' for rare kidney disease receives ODD in Korea
- by Eo, Yun-Ho Aug 12, 2025 06:16am
- A new drug for rare kidney disease, 'Vanrafia,' has been granted orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced this through notificiation board. The indication for the designation is to 'treat adult patients with primary immunoglobulin A nephropathy (IgAN) with a urine protein-to-creatinine ratio (UPCR) over 1.5g/g.' Vanrafia (atrasentan) received accelerated approval from the FDA in April. This drug is a once-daily oral non-steroidal therapeutic agent that can be used in combination with supportive therapy, including a renin-angiotensin system inhibitor. It can also be used in combination with an SGLT-2 inhibitor. The efficacy of Vanrafia was proven through an interim analysis of the Phase 3 ALIGN study. The study has not yet proven whether Vanrafia can slow the decline of kidney function in patients with IgAN. However, in the ALIGN study, patients who received Vanrafia in combination with RAS inhibitor had a clinically and statistically significant 36.1% reduction in proteinuria compared to the placebo group. These results were observed as early as week 6 and were sustained for 36 weeks. The effect of Vanrafia on UPCR was consistent across all subgroups in the primary study cohort, including those with different baseline disease characteristics such as age, gender, race, eGFR, and proteinuria. Meanwhile, IgAN is a progressive, rare autoimmune kidney disease where the immune system attacks the kidneys, often causing glomerular inflammation and proteinuria. Up to 50% of IgAN patients with persistent proteinuria progress to kidney failure within 10-20 years of diagnosis, requiring maintenance dialysis and kidney transplantation. The response to treatment can be varied.
- Opinion
- [Reporter's View] Sales dilemma of newly listed bioventures
- by Whang, byung-woo Aug 12, 2025 06:15am
- “Off to a fair start” was the market’s assessment regarding the stock prices and performance of biotech companies that went public on the KOSDAQ this year. However, upon closer inspection, some point out that the strengthened scrutiny of financial figures following the Fadu incident (accounting and revenue recognition controversy) has increased the importance of sales indicators, resulting in an ‘optical illusion’ that the listed samples generated favorable results. While there's a prevailing view that the bio industry is experiencing a positive wind of change, there's also a conflicting view that primarily companies that were inherently favorable to the industry are going public. Not all bio companies that have gone public have relied solely on sales. Some have proven their success through clinical progress or partnerships. Nevertheless, the consensus within the industry is that the growing influence of sales metrics is undeniable. In fact, sales are the most intuitive defense mechanism from an investor protection perspective. However, the biotech industry inherently has a longer time horizon than other industries. Research and development, clinical trials, regulatory approvals, and technology transfer negotiations may signal future sales, but they are difficult to fully capture in current income statements. This gap could fuel concerns about more conservative reviews and demand forecasts for R&D-focused companies. In other words, caution is needed in interpreting the current “good performance” as a structural recovery. As post-listing management requirements and external pressures increase, some are discussing attempts to strengthen the company's external image, which is not closely related to its core business. This is evaluated as a potential distraction from the company's core business and an opportunity cost that could ultimately slow down the pipeline development process. Of course, considering cases where some companies have received delisting warnings, it is difficult to blame measures that raise the threshold for new listings based on sales. The need for investor protection is clear. However, considering the purpose of technology-based special listings, the side effect of reducing opportunities for pure new drug development companies that do not generate immediate sales is ongoing. This is not unrelated to the phenomenon of using ancillary businesses to offset sales to meet sales targets. The reason why newly listed biotech companies seem to be performing well this year is due to the sales-centric filter. While this is not inherently problematic, it does not fully reflect the pace of the biotech industry. Ultimately, it is necessary to adjust the balance between regulations and evaluations to avoid overly narrowing the pathway for new drug development companies. Sales metrics are important for investor protection and corporate continuity. However, considering the purpose of the technology-based listing exemption and the unique characteristics of the biotech industry, a perspective that “also” considers sales may be more just.
- Company
- US Biosecurity Act passing is ramping up
- by Kim, Jin-Gu Aug 12, 2025 06:14am
- The U.S. Congress is re-pursuing the Biosecurity Act, which failed to pass last year. The bill explains a comprehensive set of sanctions against companies designated as 'biotechnology companies of concern.' Analysis suggests that the bill's chances of passing have increased, as it addresses the procedural issues that were a stumbling block last year. The pharmaceutical industry anticipates that if this bill, which targets Chinese biotech companies, passes, Korean biotech companies could benefit from it. According to the Korea Biotechnology Industry Organization on August 11, Senators Bill Hagerty (Republican) and Gary Peters (Democrat) recently submitted an amendment to the National Defense Authorization Act to the Senate. This amendment incorporates the contents of the Biosecurity Act that were not passed last year. The core of the bill is similar to last year's version. The U.S. administration would be able to designate 'biotechnology companies of concern.' These designated companies would be restricted from federal procurement, contracts, loans, and grants within the U.S. Specifically, U.S. government agencies would be prohibited from procuring or acquiring biotech equipment and services from designated companies. They could not enter into, extend, or renew contracts for equipment and services produced or provided by these companies. Loans and grants could not be used to procure, acquire, or use equipment and services from these companies. However, the restrictions on equipment and services produced or provided under existing contracts would be deferred for five years. The Biosecurity Act was previously pursued by the U.S. Congress last year but failed. This was due to issues raised during the legislative process concerning the lack of transparency in the designation procedure for 'biotechnology companies of concern.' At the time, five Chinese biotech companies, including WuXi Biologics, were identified as targets for regulation, leading to criticism that the process of how they were specifically designated was unclear. The absence of a procedure for removing a company from the list of concern was also a point of criticism. The newly proposed bill aims to address these concerns. The bill explains that if a company is designated as a 'biotechnology company of concern,' the U.S. government must: ▲Notify the company of its designation ▲Provide the reasons for the designation to the extent consistent with national security and law enforcement interests ▲Allow the company to submit arguments opposing the designation within 90 days of receiving the notice ▲Explain the relevant rules and procedures ▲Inform the company about the process for rescinding the designation. These measures are being pursued not as a standalone bill but as an amendment to the NDAA. It would create a new 'SEC. 881' at the end of Title VIII, Subtitle E of the NDAA, specifically 'prohibiting contracts with certain biotech providers.' If the bill passes, the U.S. Office of Management and Budget (OMB) must publish a list of 'biotechnology companies of concern' within one year of the NDAA's enactment. The designated companies would be Chinese military companies operating in the U.S. that the Department of Defense publishes annually in the Federal Register. Also, the targets include: ▲Institutes that are subject to the administrative governance structure, direction, or control of, or are operated on behalf of, a government of a foreign adversary ▲Institutes involved in some capacity in the manufacturing, distribution, provision, or procurement of biotech equipment and services; and ▲Institutes that pose a risk to national security. The subsidiaries, parent companies, and affiliates of these entities would also be included. Industry analysis suggests that the bill's chances of passing are higher than last year's, as the procedure for designating and delisting 'biotechnology companies of concern' has been improved. It is also anticipated that the affected companies will mount a more intense backlash and lobbying effort. In the U.S., it is anticipated that the Senate could begin deliberating the bill as early as this September. If the bill passes, Korean biotech companies are expected to benefit. It is anticipated to be a significant opportunity for the overseas expansion of Korean Contract Development and Manufacturing Organization (CDMO) companies. However, concerns are also being raised that Korean companies could be negatively impacted, as many domestic firms currently collaborate with the Chinese companies in question. There is apprehension that companies working with firms like WuXi Biologics and WuXi AppTec could face disruptions to their business operations. Additionally, concerns have arisen that a strategy must be developed to differentiate Korea from other countries that also seek to fill the gap left by China. While China's absence presents a clear opportunity for Korea, the same holds for other countries, such as Japan and India. Consequently, they must consider ways to win the competition against these rivals.