- LOGIN
- MemberShip
- 2025-12-17 17:12:12
- Company
- Will the Indian API cause problems?
- by Kim, Jin-Gu Oct 29, 2025 06:12am
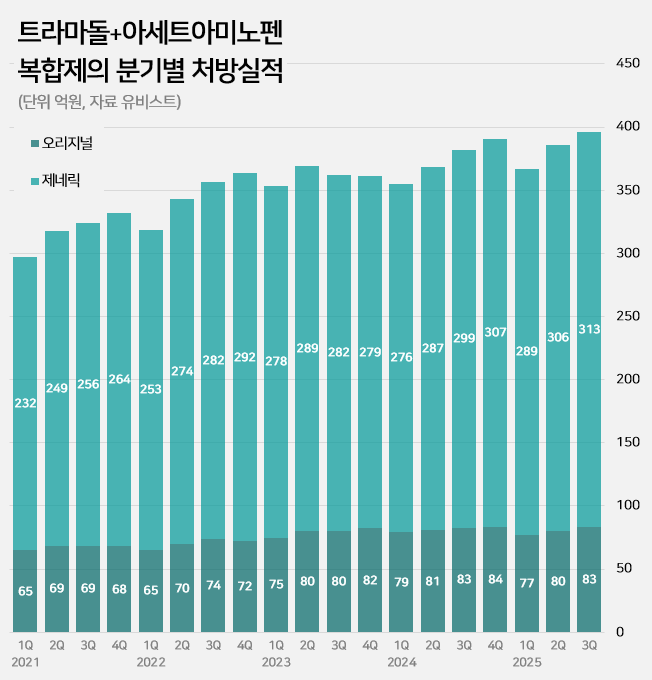
- An impurity issue has emerged as a major variable in the tramadol-acetaminophen combination drug market, valued at approximately KRW 150 billion annually. Following the detection of impurities in three generic products using Indian-sourced active pharmaceutical ingredients (API), the pharmaceutical industry is watching closely for the potential expansion of the impurity issue across all API from India. In this case, the market is expected to be reorganized around products that use non-Indian sourced API. 85% of tramadol DMFs are produced in India...Concerns Over Widespread Impurity Detection According to the Ministry of Food and Drug Safety (MFDS) on October 29, following the excess detection of the impurity NNDT (N-nitroso-desmethyl tramadol) in a tramadol monotherapy product in July, the recall target has recently been expanded to include tramadol + acetaminophen combination products. To date, impurities have been detected in three tramadol + acetaminophen combination products. The MFDS issued recall orders for specific batch number products of: ▲Dongkoo Bio & Pharma’s 'Jimuradol Tab' ▲Ausco Korea Pharma's 'Acetadol Tab' ▲Union Korea Pharm’s 'Atracen Tab.' All three products in which impurities were detected are known to have used India-sourced API. The concern is that a significant portion of the tramadol + acetaminophen combination products currently distributed in South Korea relies on API sourced from India. Currently registered sources of tramadol API: YELLO-India, BLUE-Israel, RED-Switzerland, GREEN-Korea Indeed, examining the Drug Master File DMF status for tramadol-related API shows that 62 of the 73 registered raw material manufacturers (85%) are located in India. The remaining 11 sites include 8 in Israel (11%), 2 in Switzerland (3%), and 1 in Korea (1%). Many domestically supplied products likely rely on Indian-sourced API. KRW 150 Billion Market...Top-selling products not at risk of impurity expected to benefit The three products where impurities were detected have negligible prescription sales. According to pharmaceutical market research firm UBIST, the cumulative prescription sales for these three products combined were less than KRW 400 million in the third quarter of this year. While the market impact is currently limited, analysis suggests that market restructuring will be unavoidable if impurity detection expands across Indian-sourced API. This is because securing new raw material manufacturers outside of India is difficult, and the administrative process for changing API registration alone takes approximately six months, which would lead to a market supply gap. The tramadol + acetaminophen combination market in Korea has seen steady growth in the moderate-to-severe pain treatment sector. It increased by 24% over the last four years, from KRW 120.7 billion in 2020 to KRW 127.1 billion in 2021, KRW 138.2 billion in 2022, KRW 144.6 billion in 2023, and KRW 149.6 billion last year. Cumulative sales reached KRW 114.9 billion in the third quarter of this year. Quarterly prescription sales of tramadol-acetaminophen combination drugs (DARK GREEN: original product, LIGHT GREEN: generic products; unit: KRW 100 million, source: UBIST). The original product, Janssen Korea's Ultracet, recorded cumulative sales of KRW 24.1 billion in the third quarter, a slight decrease from KRW 24.3 billion in the third quarter of last year. Generic products collectively saw a 5% increase in the same period, rising from KRW 86.3 billion to KRW 90.9 billion. The growth of top-selling generics was particularly notable: Samjin Pharm's 'Synerjet' grew by 23%, from KRW 6.9 billion to KRW 8.6 billion; Myung Moon's 'Traphen' grew from KRW 5.2 billion to KRW 6.6 billion; and Genuone Sciences' 'Painless' increased from KRW 4.4 billion to KRW 5.1 billion. In this circumstance, if the impurity issue spreads, non-detected products are expected to quickly replace the market. Currently, the market-leading products are understood to be not at risk. The original product, Ultracet, has registered a Swiss manufacturer as its API supplier. The top generic product, Samjin Pharm's Synerjet, manufactures its finished drug in-house using an Israeli-sourced API. Neither of these two products was requested to submit data in the MFDS's comprehensive investigation of NNDT impurities in tramadol.
- Company
- Fruzaqla expands its role to late-stage colorectal cancer
- by Son, Hyung Min Oct 28, 2025 06:12am
- A new treatment option has been added to the field of metastatic colorectal cancer, which is classified as a refractory condition. Takeda Korea held a press conference on the 27th at the Plaza Hotel in Jung-gu, Seoul, to commemorate the expanded indication of its colorectal cancer drug, Fruzaqla (fruquintinib). Approved in March as a fourth-line therapy, Fruzaqla was approved to extend its indication as a third-line treatment within just 6 months, becoming one of the key success cases of the Ministry of Food and Drug Safety’s fast-track program (Global Innovative drugs on Fast Track). Clinical evidence for Fruzaqla was established through the global Phase III FRESCO trial involving 416 patients with metastatic colorectal cancer. Results showed the median overall survival (OS) in the Fruzaqla group was 9.3 months, extending survival by 2.7 months compared to 6.6 months in the placebo group. The risk of death decreased by 35%, and adverse events were mostly predictable or manageable. Professor Dong-Hoe Koo of the Department of Medical Oncology at Kangbuk Samsung Hospital These results were published in major international journals such as The New England Journal of Medicine (NEJM) and subsequently recommended as Category 2A in the NCCN Guidelines and Grade I, A in the European Society for Medical Oncology (ESMO) guidelines. Professor Dong-Hoe Koo of the Department of Medical Oncology at Kangbuk Samsung Hospital stated, "Fruzaqla has high drug specificity as it does not target unnecessary targets. This enables efficient VEGFR inhibition and sustained drug exposure. Its potential for combination therapy with existing agents is also worth exploring in future clinical studies.“ He further stated, ”The survival benefit of existing treatments had not been substantial compared to the untreated group. Fruzaqla has shown real-world efficacy comparable to clinical trials, suggesting broader clinical utility.” Still high unmet need...“Expectations for improved survival in late-stage patients” South Korea's colorectal cancer incidence rate was 61.1 per 100,000 population in 2022, the second highest after thyroid cancer. Particularly concerning is the incidence rate among individuals under 40, which reached 12.9 per 100,000, the highest in the world. Colorectal cancer is a malignant tumor that develops in the colon or rectum, classified as colon cancer or rectal cancer based on location. Metastatic colorectal cancer refers to tumors that have spread beyond the primary site to other organs. Because the liver is where blood and lymph from the colon converge, it is the most common site of metastasis, and liver metastasis ranks among the leading causes of death in colorectal cancer patients. However, treatment options remain limited compared to other cancer types. Professor Myung-ah Lee of the Department of Medical Oncology at Seoul St. Mary Currently, in metastatic colorectal cancer treatment, after the first-line therapy ‘Nexavar (sorafenib)’ by Bayer, the standard second-line therapies primarily involve ‘Stivarga (regorafenib)’ by Bayer and ‘Avastin (bevacizumab)’ by Roche in combination with Lonsurf (trifluridine/tipiracil) by Taiho Pharmaceutical in Japan. However, as treatment options beyond the third line have been limited, Fruzaqla’ is expected to play a significant role in the treatment space. Professor Myung-ah Lee of the Department of Medical Oncology at Seoul St. Mary's Hospital stated, “Although several novel targeted therapies have emerged, treatment options remain insufficient beyond the third line. Due to the lack of expected efficacy, the difference in survival benefit has been minimal. Stivarga and Lonsurf have not demonstrated the expected level of effectiveness.” “Patients undergoing third-line or later treatment have already been exposed to numerous anticancer drugs, leading to a diminished quality of life. This is largely linked to the toxicity issues of these drugs. For instance, despite being a targeted therapy, Stivarga had a high incidence of adverse reactions that negatively impact quality of life. Given the shortage of drugs that are both less toxic and effective, Fruzaqla is expected to play a greater role.”
- Company
- Hanmi "Phase 3 trial confirms efficacy of GLP-1 drug"
- by Cha, Jihyun Oct 28, 2025 06:11am
- Efpeglenatide is a GLP-1 drug using Hanmi Pharmaceutical Hanmi Pharmaceutical announced on October 27 that its glucagon-like peptide-1 (GLP-1) obesity treatment candidate, 'HM11260C' (efpeglenatide), met primary endpoints in a Phase 3 top-line study in Korea. The results confirmed significant weight-loss efficacy and safety compared with placebo. This Phase 3 trial involved 448 non-diabetic adult patients with obesity. The study aimed to evaluate the superiority of efpeglenatide over placebo in terms of the mean percentage change in body weight and the percentage of subjects achieving 5% weight loss at 40 weeks. According to Hanmi Pharmaceutical, the 40-week analysis showed a statistically significant difference against placebo. The proportion of subjects achieving 5% weight loss was 79.42% in the efpeglenatide group versus 14.49% in the placebo group. The mean percent weight change was -9.75% in the efpeglenatide group versus -0.95% in the placebo group, confirming a least-squares mean difference of -8.13% between the groups. In terms of safety, gastrointestinal adverse events were reported but generally manageable: nausea 16.72% (5.37% with placebo), vomiting 11.71% (2.01% with placebo), and diarrhea 17.73% (4.70% with placebo). Hanmi Pharmaceutical plans to apply for marketing authorization with the Ministry of Food and Drug Safety (MFDS) for efpeglenatide within this year. The company explained, "Although the clinical trial involves dosing and observation up to 64 weeks, we are disclosing the 40-week interim top-line data with a plan for a submission within the year," and added, "We expect to derive improved indicators from continued dosing beyond the data announced now." Efpeglenatide is a GLP-1 drug using Hanmi Pharmaceutical's long-acting platform, 'LAPSCOVERY,' and is the most advanced clinical-stage candidate in the company's obesity and metabolic disease pipeline. It is also the fastest-developing obesity treatment among domestic Korean companies. Hanmi Pharmaceutical initially out-licensed efpeglenatide to Sanofi for diabetes in 2015 for up to €3.9 billion (approximately KRW 5.597 trillion). However, Sanofi returned the rights to the pipeline in June 2020, citing a change in business strategy. In 2023, Hanmi Pharmaceutical officially announced its decision to develop efpeglenatide, initially developed for diabetes, as an obesity treatment, mirroring the strategies of Novo Nordisk and Eli Lilly in repurposing their diabetes drugs. The core of Hanmi's strategy is the creation of a 'Korean GLP-1 Obesity Drug'. The company intends to develop efpeglenatide as a GLP-1 obesity treatment tailored to the characteristics of the Korean obese population, which has a lower proportion of severely obese patients. In a global market where Novo Nordisk and Eli Lilly have established overwhelming dominance, Hanmi Pharmaceutical aims to differentiate through price competitiveness and a stable supply chain. Kim Na-young, Executive Director of Hanmi Pharmaceutical's New Product Development Division, who led the study, stated, "Efpeglenatide, a drug the late founder of Hanmi Group, Lim Sung-ki, was passionate about, has taken a big step toward commercialization as a 'national obesity drug' by yielding effective and safe clinical results in a Korean population study," and concluded, "Efpeglenatide will become a new drug demonstrating diverse potential in the metabolic disease sector, spanning from obesity to diabetes."
- Company
- ADC offers new hope in TNBC treatment gap
- by Hwang, byoung woo Oct 28, 2025 06:11am
- The TROPION-Breast02 study presented at the European Society for Medical Oncology Congress 2025 (ESMO 2025) marks a new turning point in the treatment of triple-negative breast cancer (TNBC). As the first antibody-drug conjugate (ADC) trial to significantly improve both overall survival (OS) and progression-free survival (PFS) in patients who cannot receive immunotherapy, it heralds a change in actual clinical practice. At ESMO 2025, Professor Kyung-Hae Jung of Asan Medical Center emphasized that this study provides the first clinical evidence of survival benefit in a patient population that previously had no options beyond cytotoxic chemotherapy. First Phase III study to improve Overall Survival...beyond cytotoxic chemotherapy dependence Professor Kyung-Hae Jung of Asan Medical CenterTROPION-Breast02 is a Phase III clinical trial comparing Datroway(Dato-DXd) monotherapy with chemotherapy in 644 patients with previously untreated locally recurrent, unresectable, or metastatic triple-negative breast cancer. Progression-free survival (PFS) was 10.8 months in the Datroway group, reducing the risk of disease progression or death by 43% compared to the chemotherapy group (5.6 months) (HR 0.57, p
- Company
- Ultomiris's reimb standards eased for aHUS
- by Son, Hyung Min Oct 27, 2025 06:11am
- Prof. Myung-Gyu Kim of Korea University Anam Hospital (Nephrology) The reimbursement criteria for C5 complement inhibitors in atypical hemolytic uremic syndrome (aHUS) have been eased this month. With this improvement, as the previous criteria had stringent reimbursement conditions, such as the requirement for prior review, patient access is expected to improve. On the 24th, AstraZeneca Korea held a briefing at the Westin Seoul Parnas Hotel to commemorate the relaxation of reimbursement criteria for ‘Ultomiris (ravulizumab)’ in aHUS. Ultomiris is a next-generation C5 complement inhibitor with a half-life approximately four times longer than that of the existing Soliris (eculizumab). While Soliris requires administration every 2 weeks, Ultomiris extends the dosing interval to 8 weeks, improving treatment convenience. aHUS is an acute rare disease where the immune system's complement system becomes overactivated due to a genetic defect, causing thrombotic microangiopathy. This can lead to severe damage to multiple organs, particularly the kidneys. aHUS refers to cases of hemolytic uremic syndrome that occur independently of E. coli infection. When complement component C5 is activated on bacterial surfaces, it generates a membrane attack complex that perforates cell membranes. If this normal immune system process of complement activation continues unchecked, it causes problems in vascular endothelial cells, leading to related diseases. Ultomiris has a mechanism that inhibits this process. Expanded diagnostic and administration criteria enable timely treatment Ultomiris became reimbursable by national health insurance in January for patients with aHUS accompanied by thrombotic microangiopathy (TMA) and kidney damage. Previously, treatment was restricted to patients who had received prior approval through a pre-review process. As this can cause delays leading to disease progression, clinicians have called for a switch to post-approval review to allow faster initiation of therapy. In fact, since the implementation of the aHUS pre-review system, the average reimbursement approval rate has been only 18% (based on cases reviewed from July 2018 to August 2025), often resulting in missed treatment windows. The revised regulations, effective from the 1st of this month, minimize treatment delays by broadening the diagnostic and administration requirements for aHUS patients. Experts evaluate that clarifying the evaluation standards for treatment effectiveness may help maintain treatment continuity. Improvements in Korea The revised criteria specify that TMA activation can now be confirmed if three or more of five hematologic criteria, including thrombocytopenia, are met. Furthermore, reimbursement is also granted if the patient meets the administration criteria, including ADAMTS-13 activity of 10% or higher. Even before test results are confirmed, if the platelet count is 150×10⁹/L or higher, immediate administration is possible after submitting a pre-application form, and the administered dose is covered until the review result is notified. Furthermore, a new treatment pathway has been created as it explicitly states that when a kidney transplant is performed due to end-stage renal failure caused by aHUS, coverage is granted on a case-by-case basis. The criteria for coverage of switching from Soliris to Ultomiris have also been clearly defined. Prof. Myung-Gyu Kim of Korea University Anam Hospital (Nephrology) said, “aHUS can rapidly progress to end-stage renal failure or multi-organ damage within 48 hours, making treatment timing critical to prognosis. The revised criteria reflect global best practices and strengthen evidence for continued therapy in high-risk patients, establishing an environment enabling the rapid design of treatment strategies.”
- Company
- ImmuneOncia speeds commercialization with product-fit plan
- by Hwang, byoung woo Oct 27, 2025 06:10am
- ImmuneOncia is concretizing its ‘commercialization strategy that starts with rare cancers’ as entry points for its immuno-oncology portfolio. ImmuneOncia, a subsidiary of Yuhan Corp that specializes in immuno-oncology, presented new clinical results on its PD-L1 antibody IMC-001 (danvastotug) and CD47 antibody IMC-002 at the European Society for Medical Oncology (ESMO) 2025 Congress, outlining a novel strategy for immuno-oncology drug development. Dailypharm met with Heung-Tae Kim, CEO of ImmuneOncia, in Berlin, Germany, to discuss the significance of these achievements and the company’s future roadmap. “IMC-001 demonstrates immune microenvironment shift in gastric, esophageal, and liver cancer” Heung-Tae Kim, CEO of ImmuneOnciaCEO Kim emphasized the potential of the neoadjuvant (pre-surgery) immunotherapy market at ESMO 2025. He projected that while neoadjuvant immunotherapy is an early field, it will expand as data accumulates. The ‘NeoChance Study (IMC-001)’ presented by the company at ESMO 2025 detailed the results of administering two doses of a PD-L1 antibody preoperatively to patients with resectable gastric, esophageal, and liver cancers. CEO Kim stated, “With just two doses, the tumor's immune environment completely transformed. We directly observed the histological change where tumors, which were essentially immune deserts with almost no immune cells, converted to an inflamed phenotype.” He further stated, “Immune responses were also observed in PD-L1-negative, Microsatellite Stable (MSS) patients. This could provide evidence on its broader applicability in immuno-oncology.” In the study, the 3-year progression-free survival (PFS) and overall survival (OS) rates were 93.8% and 93.8% for gastric cancer, 80.0% and 87.5% for esophageal cancer, and 86.5% and 100% for liver cancer, respectively. Notably, the company is considering expanding its strategy from two doses to three or four doses in combination therapy, as multiple researchers at ESMO advised extending its use in combination with chemotherapy. CD47 signal reinterpreted...“Innate immunity is key” Kim also highlighted the activation of innate immunity as a key differentiator of the CD47 antibody ‘IMC-002’. He explained, “IMC-002 does more than block signals; it induces innate immunity to function strongly in patient groups with tumors with weakened immune-escape mechanisms.” This drug binds near the O-glycosylation site of the CD47 protein, minimizing interaction with red blood cells. CEO Kim stated, “Its minimal hematologic toxicity is the key difference from existing drugs, as it maintains safety even during long-term administration (up to 22 months). IMC-002 simultaneously resolves the toxicity and efficacy issues that caused previous CD47 antibodies to fail.” In actual proteomics analysis, IMC-002 showed more pronounced tumor responses in patient groups exhibiting elevated innate immune-related proteins. Based on this, ImmuneOncia plans to develop predictive biomarkers for future response assessment. From left) Professor Jin-seok Ahn of the Department of Hematology and Oncology at Samsung Medical Center and Professor Sook-ryun Park of the Department of Medical Oncology at Asan Medical Center are photographed with CEO Heung-tae Kim at the ESMO2025 poster presentation site “Immuno-oncology drugs must ultimately prove themselves through commercialization” CEO Kim clearly distinguished the development paths for its two candidates. He stated, “IMC-001 targets the pre-surgery treatment market, while IMC-002 follows an out-licensing global strategy. Both are progressing under structures that concurrently pursue commercialization and export for each candidate.” CEO Kim further revealed, “We plan to seek conditional approval with NK/T-cell lymphoma, a rare cancer, as the first indication, then expand into high tumor mutational burden (TMB-high) cancers and neoadjuvant therapy.” The global PD-1/PD-L1 antibody market is projected to continue growing, and ImmuneOncia aims to enhance the presence of domestically developed immuno-oncology drugs within it. The company is currently preparing to apply for Orphan Drug Designation (ODD) for IMC-001 and is simultaneously discussing technology transfer for IMC-002 with global pharmaceutical companies. Concluding the interview, CEO Kim reflected on the limitations of biotech companies that often remain confined to licensing deals and returns, and stressed that developing new drugs actually used by patients is the company's reason for being. While expressing ambition for ImmuneOncia to pioneer the commercialization model for domestic immuno-oncology drugs, CEO Kim added, “We will become a company evaluated not by its technology alone, but by its therapies.”
- Company
- AstraZeneca set for leadership shift after 2 years
- by Eo, Yun-Ho Oct 27, 2025 06:10am
- AstraZeneca Korea sees a leadership shift after approximately two years. According to industry sources, Sehwan Chon (51), CEO of AstraZeneca Korea, will resign at the end of this month (October). Consequently, Eldana Sauran, currently a Regional Commercial Director of Oncology Unit at AstraZeneca, is expected to be appointed as the interim CEO of the Korean subsidiary, effective November 1st. Chon led AstraZeneca Korea for two years following his appointment in December 2023. Before that, he served as Business Unit Director (BUD) of the Cardiovascular, Renal, and Metabolism Business Unit at AstraZeneca Korea until August 2020. He was then promoted to lead the Indonesian subsidiary in September of that year before returning as the Korean CEO. Meanwhile, Chon, a finance expert, graduated from Korea University with a degree in Business Administration and completed his MBA at the Wharton School of the University of Pennsylvania. He also previously worked at the global accounting and consulting firm, PWC. Chon entered the pharmaceutical industry as a Finance Manager at Abbott Korea and subsequently built expertise not only in finance but also in R&D and Business Development by working at Novartis Headquarters, its U.S. subsidiary, and AstraZeneca Korea.
- Company
- "Childhood obesity is the starting point for adult diseases"
- by Son, Hyung Min Oct 27, 2025 06:09am
- Professor Kyoung-gon Kim of the Department of Family Medicine at Gachon University Gil Medical Center "Obesity is no longer just a matter of appearance. Especially in growing children and adolescents, obesity is the starting point for adult diseases like diabetes and hypertension, and a factor that determines lifelong health. It must be approached as a disease, not just simple weight management." Professor Kyoung-gon Kim of Department of Family Medicine at Gachon University Gil Medical Center, during a recent meeting with DailyPharm, emphasized that obesity is clearly a disease that requires proactive, early intervention. If neglected, it leads to risks of future cardiovascular disease, diabetes, and liver disease. The importance of treating obesity in children and adolescents, not just adults, is gaining attention. The Korean Society for the Study of Obesity (KSSO) recently released its 'Obesity Fact Sheet 2025,' which reported that while the obesity rate in children and adolescents has slightly decreased in the last five years, nearly three out of 10 are still classified as obese. According to the fact sheet, an analysis of the prevalence of overweight and obesity from 2014 to 2023 showed an increasing trend until 2020, which reversed to a decrease after 2021. The prevalence of obesity in boys increases from age 8, peaking at 28.3% at age 14. For girls, the rate increases after age 16, reaching 26.7%. The higher the parents' Body Mass Index (BMI), the higher the probability of obesity in their children; children whose parents are in obesity class 2 or higher are more than five times more likely to be obese. The problem lies with the comorbidities of obesity. There is an increasing incidence of Type 2 diabetes and adult chronic diseases among overweight and obese children and adolescents. Professor Kim stated, "Ten years ago, Type 2 diabetes in children and adolescents was rare, but recently, we see it frequently in the hospital. Most cases are caused by obesity. Since obesity starts earlier and lasts longer in childhood, it shortens healthy life expectancy and increases the risk of complications." The long-term impact of obesity is closely related to the 'timing of exposure.' If chronic diseases or other comorbidities occur during the growth period due to being overweight, there are concerns about a lifelong financial burden of medical care, reduced quality of life, and the onset of complications. Professor Kim said, "Adult obesity often occurs after other diseases are already present, but when obesity begins at a young age, the impact lasts a lifetime." He stressed, "Child and adolescent obesity is not just an aesthetic issue but a problem that leads to future risks of cardiovascular disease, diabetes, and liver disease." Professor Kim further stated, "Just as uncontrolled hypertension leads to cerebral hemorrhage, heart disease, or kidney damage, obesity itself is a disease that causes organ damage," and added, "It must be understood as a physiological abnormality, not just an appearance problem." "Obesity is not a lack of willpower…requires therapeutic approach" Professor Kim particularly emphasized that lifestyle modification alone has limitations in treating obesity. He argued that if weight loss is difficult for adults with obesity in terms of willpower, it is much harder for adolescents, who are still growing, to suppress their own appetite. Professor Kim stated, "Appetite is not a matter of willpower; it is a biological response involving the action of various neurological and hormonal substances secreted by the pituitary gland and adipose tissue," and added, "For this reason, weight loss is difficult with the 'eat less' approach." Recently, the treatment paradigm has been changing with the emergence of additional new obesity drugs, such as the Glucagon-like Peptide-1 (GLP-1) class of drugs. Consequently, the concept of treatment is gradually taking root among overweight and obese adult patients. Professor Kim assessed, "In the past, obesity treatments were centered on appetite suppression, but with the emergence of GLP-1 class drugs, we can now regulate the physiological mechanism itself. The perception of treatment among patients has also changed significantly due to recently launched treatments, and cases of actively considering drug therapy are increasing." However, the use of these drugs in the child and adolescent population is limited. Currently, only orlistat drugs, 'Saxenda (liraglutide)' and 'Wegovy (semaglutide)' are approved for use in patients aged 12 and older in Korea. On the other hand, obesity drugs approved relatively recently for adult patients, such as 'Qsymia (phentermine·topiramate)' and 'Mounjaro (tirzepatide),' are still not available for use in obese children and adolescents in Korea. Professor Kim assessed, "Government regulations are too strict. There are no approved drugs for children under 12, and the options for those over 12 are limited." He argued, "If efficacy and safety are proven through studies in Korean patients, the government must actively consider approving additional medications." In the case of some new obesity drugs like Qsymia, they are already being used for adolescent patients in the U.S. While concerns about misuse and abuse may arise, the expert view is that it is premature to make a rash judgment, given the current lack of treatment options. Professor Kim said, "Qsymia is already approved as an adolescent obesity treatment in the U.S." He said, "Considering the circumstance in Korea, the potential for adolescent misuse and abuse is low. Overly stringent regulations due to concerns about misuse are undesirable." Beyond the case of children and adolescents, the societal perception that obesity is a disease remains low. While prevention is most important in child and adolescent obesity, experts believe that proactive treatment is necessary once the disease stage has been reached. Professor Kim stated, "Drug therapy is maximized only when based on a healthy lifestyle." However, he added, "Adolescents with severe obesity or those with complications such as hypertension, elevated liver enzymes, or Type 2 diabetes need to consider drug treatment actively." Professor Kim emphasized, "For adolescents, for whom lifestyle modification is more challenging than for adults, drug therapy should be combined with education to maintain healthy eating habits and physical activity in the long term."
- Company
- ‘Reimb targeted therapies for Stage IV gastric cancer'
- by Son, Hyung Min Oct 24, 2025 06:15am
- A targeted therapy option for gastric cancer that demonstrated exceptional survival extension benefits in Asian patients is on the verge of being listed for insurance reimbursement in Korea. If reimbursed, the medical community expects the improved access to the new drug will contribute to improving the persistently low survival rates among metastatic gastric cancer patients. 아스텔라스 According to industry sources on the 24th, the Health Insurance Review and Assessment Service (HIRA) plans to convene its 8th Cancer Disease Deliberation Committee meeting later this month. Among the treatments likely to be reviewed, Astellas' gastric cancer drug ‘Vyloy (zolbetuximab)’ is a prominent candidate. Vyloy is the first new drug introduced in the gastric cancer field in 14 years since HER2-targeted therapies, and it was the world's first drug approved for patients with Claudin 18.2-positive metastatic gastric cancer. It was approved in Korea last September. The treatment is an IgG1 monoclonal antibody that binds selectively to Claudin 18.2, a protein expressed in the stomach. It works by binding to the Claudin-18.2 protein expressed on gastric epithelial tumor cells. Claudins are a type of protein that regulates the exchange of cellular molecules and maintains cell junctions. They are expressed at limited levels in healthy tissues but are overexpressed in certain solid tumors. Among these, Claudin-18.2 is known to be expressed in gastrointestinal cancers such as gastric and pancreatic cancer. This protein is expressed in approximately 38% of all gastric cancer patients, which far exceeds the HER2-positive population (10-15%). Unlike lung or breast cancer, where various targeted and immunotherapy drugs have emerged to improve survival rates, gastric cancer has long been a barren field for targeted therapy. This is because biomarkers capable of targeting metastatic gastric cancer cells were limited. This stands in stark contrast to lung cancer, where the relative survival rate at the metastatic stage more than doubled from 4.9% in 2011 to 12.9% in 2022. Until now, approximately 90% of metastatic gastric cancer patients diagnosed as HER2-negative have had virtually no available targeted therapy options. The introduction of Vyloy has provided new treatment opportunities for about 40% of patients, leading the clinical field to regard it as a paradigm shift in gastric cancer treatment. Vyloy showed exceptional survival benefit in Asian patient populations, including Koreans. According to the SPOTLIGHT clinical, which was conducted in Claudin18.2-positive, HER2-negative metastatic gastric cancer patients, the median progression-free survival (PFS) in the Vyloy group was 12.55 months for Asian patients, longer than the 9.69 months for non-Asian patients. Overall survival (OS) also exceeded that of non-Asian patients at 21.49 months versus 16.99 months. Dae-young Zang, president of the Korean Gastric Cancer Association (Department of Hemato-Oncology, Hallym University Sacred Heart Hospital), emphasized, “The Korean subgroup analysis of the pivotal study showed that median overall survival was 30 months — nearly double that of the placebo group. Vyloy represents a significant therapeutic advancement that can substantially improve survival outcomes for Korean patients with metastatic gastric cancer.” KGCA recommends Vyloy at the ‘strongest level’...reimbursement needed Reflecting this data, the Korean Gastric Cancer Association issued a ‘strong recommendation’ in January this year via its official journal JGC (Journal of Gastric Cancer) for Vyloy as a first-line therapy for HER2-negative, Claudin18.2-positive metastatic gastric cancer patients. However, Vyloy's clinical value is not being fully realized on site. This is because it is not yet reimbursed by national health insurance, leaving many patients unable to afford the high treatment costs. Metastatic gastric cancer is a disease with a pronounced survival gap based on income level. According to Statistics Korea data, the mortality risk for the lowest income group among gastric cancer patients in Korea is 1.72 times higher than that of the highest income group. This structure effectively means that the burden of treatment costs determines survival. In contrast, Japan approved the drug and granted its reimbursement simultaneously last March, with over 3,000 patients already receiving Vyloy treatment. Considering that Japan's gastric cancer incidence rate per 100,000 people is similar to Korea's (Japan: 27.6, Korea: 27), Korea's delayed coverage is leading to a clear gap in treatment accessibility. South Korea is a globally recognized leader in gastric cancer treatment. The 5-year relative survival rate for all gastric cancer patients reaches 77.5%, and for localized gastric cancer confined to the stomach, it is 97.4%, approaching a cure. As of 2022, approximately 62% of gastric cancer patients in South Korea are diagnosed at an early, localized stage, supporting this achievement. However, even within this early-diagnosis-focused structure, blind spots persist. The medical community is increasingly focusing on the low survival rates of metastatic (stage IV) gastric cancer patients. Accounting for about 10% of all gastric cancer patients, these individuals face difficulty in receiving treatment because the cancer has already spread at the time of diagnosis. The actual 5-year relative survival rate for metastatic gastric cancer patients in Korea is 7.5%, meaning only 7 out of 100 patients survive beyond 5 years. This is the lowest among the five major cancers, showing a significant gap compared to lung cancer (12.9%), colorectal cancer (20.6%), breast cancer (49%), and thyroid cancer (63.7%). The average survival period is also only about one and a half years. Zang said, “As a global leader in gastric cancer care, Korea should act swiftly to provide reimbursement for Vyloy to offer an opportunity for survival to patients and drive innovation in gastric cancer treatment worldwide.”
- Company
- Metalyse is approved for acute ischemic stroke in KOR
- by Son, Hyung Min Oct 24, 2025 06:14am
- Boehringer Ingelheim Korea (General Manager: Anna-Maria Boie) announced on the 23rd that the Ministry of Food and Drug Safety approved Metalyse (tenecteplase) as a treatment for acute ischemic stroke in adults on October 2. The approval marks the first new option introduced for acute ischemic stroke in roughly two decades, following Actilyse (alteplase), which had then expanded its indication to acute ischemic stroke in 2002. Metallase is a recombinant protein drug that contains the active ingredient tenecteplase. Tenecteplase replaces three sites in the protein structure of ‘alteplase’, the active ingredient in the existing standard treatment option for acute ischemic stroke, Actilyse. A meta-analysis of data from 11 clinical trials involving 7,545 patients worldwide showed that Metalyse demonstrated a significantly higher rate of functional recovery (mRS 0–1) at 3 months compared with Actilyse, with a comparable safety profile. Based on this evidence, the Korean Stroke Society published a scientific statement in the Journal of Clinical Neurology in July recommending Metalyse as an alternative therapeutic option to Actilyse for acute ischemic stroke. Furthermore, multiple global clinical trials have confirmed that Metalyse offers several advantages over Actilyse in terms of half-life, fibrin selectivity, and PAI-1 resistance. Metalyse’s half-life is approximately 22 minutes, which is longer-lasting than Actilyse (approximately 3.5 minutes). Its fibrin selectivity, which shows how precisely the drug acts on fibrin—the main component of thrombi—is approximately 15 times higher than existing treatments. Its resistance to PAI-1, a protein that inhibits thrombolysis in the body, is approximately 80 times higher. Ina Hwang, Executive Head of the Specialty Care Franchise at Boehringer Ingelheim Korea, stated, “With the globally validated Metalyse now available for full use in the domestic acute ischemic stroke treatment setting, we will continue to collaborate with Korean healthcare providers to ensure more patients can receive Metalyse’s rapid and effective treatment benefit.” Metalyse has been approved for acute ischemic stroke in numerous countries worldwide, including the UK, Europe, Australia, and New Zealand. In Asia, its commercial launch was recently completed in China. The European Stroke Organisation (ESO) and Canadian Stroke Best Practice Guidelines strongly recommend Metalyse as an alternative therapy to alteplase, while the Australian and New Zealand guidelines also specify Metalyse as a first-line therapy.