- LOGIN
- MemberShip
- 2025-12-22 01:22:13
- Company
- China's mAb 'Hetronifly' secures orphan drug designation
- by Eo, Yun-Ho Apr 07, 2025 05:51am
- Product photo of Hetronifly China-made immune checkpoint inhibitor has been designated as an orphan drug in South Korea. The Ministry of Food and Drug Safety (MFDS) recently announced on a notification board that it has granted Shanghai Henlius Biotech's 'Hetronifly (serplulima)' an orphan drug designation (ODD). The drug is indicated for 'use in combination with carboplatin and etoposide as a first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).' Hetronifly was initially approved in China in 2023 under the product name 'Hansizhuang.' This drug also secured approval recommendations in Europe. Hetronifly has gained attention as the first immunotherapy for cancer as a first-line treatment of ES-SCLC. Hetronifly demonstrated efficacy through the multicenter Phase 3 'ASTRUM-005' study. The study was randomized, double-blind, placebo-controlled, and global multicenter. The results showed that the patient group administered with Hetronifly had an average survival period of 15.8 months, which was significantly longer than the placebo group. The analysis suggested that the Hetronifly group's mortality rate was 38% lower than the control group. Furthermore, a subgroup of Hetronifly-administered study participants who are Asians had 4.8 months extension in average survival period. Meanwhile, Henlius Biotech was initially launched as China's top company specializing in biosimilar antibodies. The company has developed biosimilar antibodies to several antibody drugs, including breast cancer medicine 'Herceptin (trastuzumab)' and AbbVie's rheumatoid arthritis medicine 'Humira (adalimumab),' and launched across countries.
- Company
- AbbVie Korea sales up twofold in two years
- by Son, Hyung Min Apr 07, 2025 05:51am
- With its new drugs for immune diseases, AbbVie Korea's sales have reportedly increased twofold in two years. AbbVie Korea is successfully transitioning generational changes for Humira follow-up of new drugs, including Skyrizi and Rinvoq. According to the Financial Supervisory Service on April 4, AbbVie recorded KRW 308.9 billion last year, up 32% from KRW 234.7 billion in 2023. Its operational profits for the same period increased from KRW 11.5 billion to KRW 14.7 billion, up 27%. AbbVie Korea AbbVie Korea's sales have been led by Humira. Developed by the global pharmaceutical company AbbVie, Humira is a treatment for autoimmune diseases, and it was approved in the United States in 2003. Humira is indicated for 15 autoimmune disease areas, including rheumatoid arthritis, Crohn's disease, ulcerative colitis, and psoriasis. However, after the Humira patent expired, the introduction of biosimilars, follow-up biological drugs with the same indications, and Janus Kinase (JAK) inhibitors led to declining sales. According to market research firm IQVIA, Humira sales once recorded KRW 104.0 billion in 2020 and decreased to KRW 80 billion in 2022. Consequently, there haven't been significant changes to AbbVie Korea's sales. AbbVie Korea recorded KRW 146.7 billion in 2020, KRW 140.4 billion in 2021, and KRW 154.6 billion in 2022, maintaining the KRW 100 billion sales range. However, in 2023, the domestic market saw significant traction for Rinvoq and Skyrizi, contributing to a substantial increase in AbbVie Korea's sales. AbbVie Korea recorded sales of KRW 234.7 billion in 2023, a 52% year-over-year increase. For the first time last year, its sales exceeded KRW 300 billion, with last year's total of KRW 308.9 billion representing a 100% increase compared to two years ago. Rinvoq, a JAK inhibitor that selectively targets JAK1, was approved in Korea in 2020 for the treatment of rheumatoid arthritis. It subsequently gained approval in 2021 for atopic dermatitis, and in 2022 and 2023, it expanded its indications to include ulcerative colitis and Crohn's disease, respectively. Sales for Rinvoq have increased with its expanded indications. According to market research firm UBIST, Rinvoq's sales in Korea increased by 450% from KRW 1.4 billion in 2021 to KRW 7.7 billion in 2022. After surpassing KRW 10 billion in early 2023, sales reached KRW 26.1 billion last year, setting a new record. AbbVie Korea is also optimistic about the growth prospects for 'Skyrizi,' a biologic targeting interleukin (IL)-23. Approved in 2019 for plaque psoriasis, Skyrizi was approved later in 2022 for psoriatic arthritis, and last year for palmoplantar pustulosis. According to IQVIA, Skyrizi's sales climbed steeply from KRW 8.4 billion in 2021 to KRW 16.5 billion in 2022, and then to KRW 27.6 billion in 2023. As of 2023, the combined sales of Rinvoq and Skyrizi have exceeded KRW 50 billion, signaling a successful generational transition from Humira in the autoimmune disease area. A CGRP peptide new drug, AbbVie Korea also optimistic for its next-generation drugs. Last year, the company received domestic approval for 'Aquipta,' a CGRP peptide new drug related to calcitonin genes. In this area, competitors such as Eli Lilly's Emgality and Teva's Ajovy have already made tractions, but Aqipta's advantage lies in its oral formulation. Furthermore, AbbVie Korea secured domestic approval last year for its bispecific antibody new drug, 'Epkinly,' which targets CD20 and CD3 expressed in blood cancers. Epkinly is indicated for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL). In a global Phase 1/2 EPCORE NHL-1 study, Epkinly achieved an overall response rate (ORR) of 62%, with a complete response (CR) rate of 39%.
- Company
- GPP drug Spevigo can be prescribed in general hospitals
- by Eo, Yun-Ho Apr 07, 2025 05:51am
- 'Spevigo', a treatment for Generalized Pustular Psoriasis (GPP), may now be prescribed in general hospitals in Korea. According to industry sources, the new drug for generalized pustular psoriasis (GPP) from Boehringer Ingelheim Korea, Spevigo (spesolimab), has recently passed the Drug Committee (DC) of the Seoul National University Hospital. Spevigo is a humanized antagonistic monoclonal antibody that binds to the IL-36R. It prevents the subsequent activation of IL-36R and downstream activation of pro-inflammatory and pro-fibrotic pathways, providing a new treatment opportunity for GPP patients. Generalized Pustular Psoriasis (GPP) is characterized by systemic inflammation affecting the skin and internal organs. Its symptoms include diffuse erythema, fever, neutropenia, and skin pain. Spevigo is the first drug approved for GPP in Korea, and the drug is approved as a treatment for adult GPP patients who experience rapid worsening of their conditions. However, Spevigo is still not reimbursed in Korea. Spevigo was approved in Korea in August 2023, but there has been no progress in discussions on its reimbursement to date. As “generalized pustular psoriasis (KDC code L40.1)” has been included in the list of rare diseases eligible for special calculation since January 1 last year, there is also interest in whether the only treatment option, 'Spevigo', will be listed for reimbursement in Korea. Meanwhile, the company is accumulating data on the efficacy and safety of Spevigo in GPP patients through the Phase II Effisayil-1/2 trial and the Effisayil-ON, a five-year open-label extension study (OLE), and the Effisayil-REP study for patients with recurrent exacerbations. The results of the Phase II Effisayil 1 study, a 12-week clinical trial of 53 GPP patients who experienced flare-ups, showed that the percentage of patients who did not have visible pustules at the first week of treatment was 54% in the Spevigo group, compared to 6% in the placebo group, confirming the drug’s rapid elimination of pustules. The percentage of patients with completely or almost clear skin (GPPGA score of 0 or 1) was also 43% in the Spevigo group and 11% in the placebo group, confirming the significant skin symptom improvement effect of Spevigo. These effects of pustule removal and symptom improvement were consistent across all subgroups, regardless of gender, disease severity, race, or BMI. In addition, the time to GPP flare was determined in the first and largest Phase IIb clinical trial, the Effisayil 2 study, which evaluated the efficacy and safety of GPP flare prevention in 123 patients with GPP aged 12-75. The results showed that the high-dose Spevigo group’s flare improved by 84% compared to the placebo group.
- Policy
- Bylvay to be redeliberated for reimbursement in Korea
- by Lee, Tak-Sun Apr 07, 2025 05:51am
- Ipsen Korea’s Bylvay Cap, which has been undergoing an accelerated listing process as the first drug included in the pilot project for the parallel operation of the approval-benefit evaluation-drug price negotiation system, has failed to receive recognition as being eligible for reimbursement by the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service (HIRA). However, DREC’s result - 'redeliberation - has allowed the company to reattempt review at the next DREC meeting. HIRA announced on the 3rd that it will hold the 4th 2025 DREC meeting and redeliberate the reimbursement of Bylvay Cap. Ipsen Korea’s Bylvay Cap is a drug used to treat pruritus in patients with progressive familial intrahepatic cholestasis (PFIC), and it has attracted attention as the first drug selected for the pilot project for the parallel operation of the ‘approval-assessment-negotiation linkage’ system. The pilot project for the parallel operation of the ‘approval-assessment-negotiation linkage’ system was introduced to shorten the period from drug approval to reimbursement, and as the name suggests, the approval, evaluation, and negotiation process for a single drug is carried out all at once to shorten the period of listing. However, the evaluation stage has slowed down the process. The reimbursement of another drug included in the pilot project, Qarziba Inj, was also once delayed at the DREC review stage. Qarziba has been reimbursed by health insurance since December last year, but Bylvay has yet to pass the DREC review process. Last month, HIRA said that it was conducting an in-depth review of the clinical usefulness and cost-effectiveness of Bylvay after gathering the opinions of relevant experts and academic societies, saying that an assessment of the adequacy of reimbursement was underway. Some academics have expressed concern that not only is the speed of listing Bylvay Cap problematic but that the indications under reimbursement review are also narrow. On this day, the results of the deliberation on the Leqvio pre-filled syringe (inclisiran, Novartis Korea), which was on the agenda with the Bylvay Cap, showed that the reimbursement was appropriate if the company accepted an amount lower than the assessed amount. Accordingly, if Novartis accepts the assessed amount, the matter will move on to the negotiation stage with the National Health Insurance Service.
- Policy
- Gov’t works to stop the shortage of essential drugs
- by Lee, Jeong-Hwan Apr 07, 2025 05:50am
- The government will come up with improvement measures within this year to eradicate and alleviate the instability in the supply of national essential drugs, which has been occurring frequently since the COVID-19 pandemic. The government plans to begin the groundwork for overhauling the entire system, including the method for designating national essential drugs, the operation of the council of relevant government ministries and agencies, and government policy support and preferential measures for stable supply. This is done by examining the current status of the essential drug systems in major countries overseas and seeking ways to improve the stability of supply based on an analysis of the current status of Korea’s operation of the essential medicine system. On the 6th, the Ministry of Food and Drug Safety announced that it would begin research on ways to collaborate on the classification and stable supply of essential medicines. This is a response to the growing risk of health security crises and the increasing frequency of shortages of essential drugs such as cold medicines that arose as countries around the world have adopted a national drug policy centered on their own countries since the COVID-19 pandemic. Since 2017, the government has designated and managed drugs that are essential for medical sites such as disease control and radiation disaster prevention, and therefore must be supplied stably as national essential medicines. The Minister of Health and Welfare and the Minister of Food and Drug Safety designate the essential medicines in consultation with the heads of relevant central administrative agencies. However, as front-line pharmacies are constantly experiencing difficulties in obtaining essential medicines, citizens are repeatedly unable to purchase essential drugs such as cold medicine and fever reducers when they need them. The Ministry of Food and Drug Safety examines the purpose of the essential medicine system in advanced pharmaceutical countries and investigates the classification method by type, such as the nature of the product and its intended use. The plan is to look into the current state of the system, including the selection criteria, procedures, and collection of advisory opinions, and analyze the current use of policy support and preferential measures for the stable supply of essential medicines, as well as the current state of operation of organizations such as selection and advisory councils and committees. Based on this, the research team will seek direction for the advancement of Korea's national essential drug policy. As the number of national essential medicines that were first designated around government stockpiles has gradually increased to include items that require a stable supply in the private medical field, the overall goal is to find improvement measures that reflect the characteristics of each national essential medicine. Afterward, the government will prepare a plan to improve the criteria for designating national essential medicines, review the classification plan by use, and come up with a plan to reorganize the list, as well as an operational plan to maintain its suitability in the field. The research will comprehensively review the necessity of each product in the product group when designating and re-evaluating national essential medicines. In addition, it will consider the need to distinguish the system from similar systems and review the scope of designation and exclusion of national essential medicines. Ultimately, it will establish a plan for reorganizing and operating the Stable Supply Council and Sub-Council to facilitate discussions on the designation of national essential medicines and stable supply. The MFDS explained, “We will identify the government policy tools and the collaboration needs with relevant ministries and agencies that can be used to enable the council to determine and implement policies for the stable supply of essential medicines. We will also identify the improvements needed to strengthen the capacity to support the stable supply of national essential medicines, and find ways to strengthen the human and material infrastructure for this.”
- Company
- K-pharma trials show greenlight overcoming bile duct cancer
- by Son, Hyung Min Apr 04, 2025 05:58am
- South Korean and the overseas pharmaceutical industry's candidate products that are targeted anticancer agents for bile duct cancer (cholangiocarcinoma) are showing results of effectiveness, indicating the potential for commercialization possibilities. Korean and overseas pharmaceutical companies, including Handok's US partner Compass Therapeutics and HLB, are challenging this area. Compass "tovecimig met primary endpoint in global clinical trials" #iAccording to industry sources on April 3, US-based Compass announced top-line results from tovecimig's COMPANION-002 Phase 2/3 trial evaluating patients with metastatic or recurrent cholangiocarcinoma. Tovecimig is a novel drug candidate for cholangiocarcinoma, which was developed by the Korean company ABL Bio. Handok holds domestic commercialization rights, and Compass holds global rights. This new drug candidate is a dual antibody that simultaneously targets delta-like ligand 4 (DLL4) and vascular endothelial growth factor (VEGF), thereby inducing neovascularization in the tumor microenvironment. The study enrolled 168 adult patients with metastatic or recurrent cholangiocarcinoma and compare the efficacy and safety of the tovecimig+paclitaxel combination therapy versus paclitaxel monotherapy. The clinical trial results showed that the primary endpoint, objective response rate (ORR), was 17.1% in the tovecimig+paclitaxel group, compared to only 5.3% in the paclitaxel monotherapy group. Furthermore, the incidence of progressive disease (PD) was 16.2% in the combination group versus 42.1% in the monotherapy group. Regarding safety, Grade ≥3 adverse events observed in the Phase 2 trial were consistent with previous studies. These included neutropenia (50%), hypertension (16.7%), anemia (12.5%), and thrombocytopenia (8.3%). There was one reported case of Grade 5 pneumonia, and 25% of patients discontinued treatment due to adverse events such as confusion, pulmonary embolism, and elevated blood creatinine levels. Handok plans to utilize these results as supporting data for regulatory approval of tovecimig in Korea. Following these top-line findings, Compass intends to present additional data, including key secondary endpoints, from the COMPANION-002 trial later this quarter. Compass also supports a researcher-led clinical trial evaluating tovecimig as a first-line therapy for cholangiocarcinoma, in addition to the ongoing COMPANION-002 study. This trial, led by the MD Anderson Cancer Center at the University of Texas, investigates the efficacy and safety of adding tovecimig to the standard regimen of Imfinzi plus chemotherapy. Rivoceranib shows potential in cholangiocarcinoma Cholangiocarcinoma is considered one of the most challenging solid tumors due to its low survival rate and the limited availability of new targeted therapies. Although the patient population is relatively small compared to other cancers, early diagnosis remains difficult, and the disease is characterized by rapid metastasis and recurrence to surrounding organs, resulting in a 5-year relative survival rate of only 28.9% (2017–2021). Reports indicate that 7 out of 10 cholangiocarcinoma patients eventually die, and the domestic mortality rate in Korea is estimated at 11.6%. Another reason for low survival rate is the scarcity of effective treatment options. For patients with locally advanced or metastatic cholangiocarcinoma who are not candidates for surgery and have failed first-line therapy, there is a critical lack of second-line options. Although the development of various targeted therapies was possible for cholangiocarcinoma, like lung cancer, limited patient numbers have restricted research and investment. However, growing interest from the pharmaceutical industry is now yielding promising research outcomes. HandokHandok has recently expanded the therapeutic landscape by introducing two targeted treatments in Korea: its FGFR2-targeted therapy 'Pemzayre' and Servier’s IDH1-targeted therapy 'Tipsovo.' Other Korean and overseas companies are venturing into this field. FGFR genetic abnormalities are known to contribute not only to cancer cell proliferation, survival, and migration but also to tumor angiogenesis and drug resistance. Meanwhile, IDH1 mutations are predominantly found in gliomas and cholangiocarcinomas, with a particular prevalence in intrahepatic cholangiocarcinoma. In addition to Handok, Korean company HLB is actively investigating new treatment options for cholangiocarcinoma. HLB, in collaboration with Hangseo Pharmaceuticals, is evaluating the clinical efficacy of a combination regimen comprising the VEGFR2 inhibitor riboceranib and the immuno-oncology agent camrelizumab in several solid tumors, including liver cancer and cholangiocarcinoma. In a clinical study conducted over approximately two years beginning in January 2021, 28 patients with advanced cholangiocarcinoma were treated with riboceranib combination therapy, administered either as a first-line or second-line treatment. The trial demonstrated a median overall survival (OS) of 12.8 months and a median progression-free survival (PFS) of 6.3 months, nearly double the typical 6- to 7-month survival period seen in patients with inoperable cholangiocarcinoma. Notably, among patients who received riboceranib combination therapy as first-line treatment, the ORR was 50.0%. HLB plans to review additional investigator-led clinical data from this study to further expand its pipeline. New drug R&D for cholangiocarcinoma is active globally China's TransThera Sciences is currently conducting a Phase 3 clinical trial in cholangiocarcinoma patients, with studies underway not only domestically but also in the United States, the United Kingdom, China, and other regions. Tinengotinib is a next-generation FGFR inhibitor designed for advanced cholangiocarcinoma patients harboring FGFR mutations who have prior treatment history. According to TransThera Sciences, this multi-kinase inhibitor features a unique FGFR binding mechanism that bypasses acquired resistance pathways. Clinical trial results showed that tinengotinib is effective in patients with advanced cholangiocarcinoma harboring FGFR mutations who have previously received systemic chemotherapy. In the clinical trial, patients were categorized into four groups based on their FGFR mutation status and treatment history. ▲Group A1 (13 patients): Patients with FGFR2 fusions whose disease progressed following treatment with conventional FGFR inhibitors ▲Group A2 (10 patients): Patients with FGFR2 fusions who initially responded to conventional FGFR inhibitors but subsequently relapsed ▲Group B (12 patients): Patients with non-fusion FGFR mutations ▲Group C (13 patients): Patients with no FGFR mutations (FGFRwt). Eisai has recently launched its FGFR2 inhibitor, Tasfygo, in Japan, boldly entering the cholangiocarcinoma market. In a Phase 2 study, Tasfygo achieved an ORR) of 30.2% (90% CI: 20.7–41.0), statistically surpassing the pre-set tumor response threshold of 15%. Meanwhile, U.S.-based Jazz Pharmaceuticals plans to launch its HER2-targeted therapy, 'Ziihera,' globally. Jazz Pharmaceuticals secured the development rights for Ziihera from U.S. biotech company Zymeworks in 2022 and has been conducting clinical studies. Accelerated approval was granted for this therapy in the U.S. last November, and it is now positioned to expand into international markets, including Korea. Its basis of approval HERIZON-BTC-01, Ziihera demonstrated an ORR of 52% with a duration of response (DOR) of 14.9 months.
- Company
- Obesity drug craze affects global pharma subsidiaries in KOR
- by Son, Hyung Min Apr 04, 2025 05:58am
- Whether or not a new obesity drug in the glucagon-like peptide (GLP-1) family has been launched has had a significant impact on the performance of Novo Nordisk and Lilly Korea. Last year, Novo Nordisk’s sales increased by 63% upon the launch of Wegovy in the domestic market. In the case of Lilly Korea, sales decreased slightly due to the delay in the launch of the licensed Mounjaro into the market in 2023 and the sluggish sales of some other products. According to the Financial Supervisory Service on the 3rd, Novo Nordisk Korea's sales increased 63% from KRW 230.2 billion in 2023 to KRW 374.7 billion last year. Operating profit increased 65% from KRW 8.3 billion to KRW 13.7 billion over the same period. Novo Nordisk’s sales growth was driven by Wegovy. According to the market research institution IQVIA, Wegovy recorded sales of KRW 60.3 billion in the same quarter since its launch in October last year. Wegovy, which was approved in Korea in April 2023, is a GLP-1 formulation composed of semaglutide, which has been confirmed to have effects on reducing weight and glycated hemoglobin. Novo Nordisk developed the obesity treatment drug Wegovy, a semaglutide that confirmed the weight loss effect of patients during the clinical trial of its GLP-1 class diabetes drug candidate and is administered once a week. Novo Nordisk and Lilly Korea Novo Nordisk’s insulin product also contributed to the growth in its sales. According to the market research institution UBIST, sales of its combination of liraglutide, a GLP-1 analogue, and the insulin degludec, Xultophy, reached KRW 15.1 billion last year, up 26% from the previous year. In addition, the once-weekly insulin product Tresiba and the insulin combination product Ryzodeg also recorded sales of KRW 38 billion and KRW 31.3 billion last year, up 3% and 7%, respectively. Novo Nordisk plans to further strengthen its position in the field of obesity. Currently, Novo Nordisk is conducting a global Phase III clinical trial of its new obesity drug, ‘CagriSema,' following the success of its previous drugs, Wegovy and Saxenda. CagriSema is a combination of 2.4 mg of semaglutide, the main ingredient of Wegovy, and 2.4 mg of the long-acting amylin analogue, Cagrilintide. This drug is regarded as a next-generation obesity drug. In clinical trials, CagriSema has been shown to be 23% more effective in weight loss than existing single-agent semaglutide. The expected end of clinical trials is in the first quarter of next year, after which Novo Nordisk plans to apply for approval from major regulatory agencies around the world. Lilly shows slow performance due to non-launch of Mounjaro On the other hand, the performance of Lilly Korea’s Mounjaro, which is also a GLP-1 class diabetes and obesity drug, showed a slight decline. Lilly Korea recorded sales of KRW 164.2 billion last year, down 2% from KRW 167.8 billion the previous year. Operating profit was KRW 10.3 billion, down 1% from 2023. In the case of Lilly Korea, it is analyzed that sales have not increased significantly due to the delay in the launch of Mounjaro. Mounjaro is a new diabetes drug developed by Lilly. Mounjaro acts on both the gastric inhibitory peptide (GIP) receptor and the GLP-1 receptor to promote insulin secretion, improve insulin resistance, and reduce glucagon secretion, thereby reducing blood sugar levels before and after meals. Obesity drugs Wegovy, Zepbound, Saxenda Mounjaro has the advantage of not only controlling blood sugar levels but also having an excellent weight loss effect. Mounjaro has proven its weight loss effect through the results of the Phase III SURMOUNT-1 clinical trial, which was conducted on overweight adult patients who are not diabetic, have a body mass index (BMI) of 30 kg/m2 or higher, or have one or more comorbidities, and who were administered Mounjaro once a week. Lilly launched the same-ingredient obesity treatment, Zepbound, in the US market in November 2023, as it has confirmed the weight loss effect in the clinical trial of the drug in the US. In Korea, the drug was approved as a treatment for diabetes in June 2023 and secured additional indications as a new obesity drug with the same product name in August last year. However, its launch in the domestic market has not yet taken place. In addition, sales of Lilly Korea’s Trulicity and Cymbalta were also sluggish last year. Sales of Trulicity, a GLP-1 class diabetes drug, fell 16% from KRW 44.4 billion the previous year to KRW 37.2 billion. Sales of Cymbalta, an antidepressant, fell 52% from 2023 to KRW 5.1 billion. Lilly Korea is looking forward to the success of the SGLT-2 inhibitor Jardiance. Jardiance's sales last year rose 14% year-on-year to KRW 66.3 billion. It benefited from the withdrawal of its rival product, Forxiga, from the market last year. In addition, the company is aiming for a rebound by launching Ebglyss, a new atopic dermatitis drug, in January this year. Lilly is also preparing a next-generation diabetes and obesity drug. Retatrutide, which is being developed by Lilly, is a next-generation diabetes and obesity drug that acts on three receptors: GLP-1, GIP, and GCG (glucagon). To date, no new obesity drugs have been commercialized using this mechanism. In the Phase II clinical trial, Retatrutide demonstrated a weight loss effect of 22.8% and 24.2% when administered at 8 mg and 12 mg at week 48, respectively. This is a higher weight loss effect than the 20.2% of the existing GLP-1 and GIP targeting Mounjaro. Currently, Lilly is confirming the potential of retatrutide not only in obesity but also in various chronic diseases such as diabetes and liver disease.
- Company
- New drug 'Niktimvo' for cGVHD receives ODD in Korea
- by Eo, Yun-Ho Apr 04, 2025 05:58am
- 'Niktimvo,' a new drug for the treatment of chronic graft-versus-host disease (cGVHD), has been designated as an orphan drug in South Korea. The Ministry of Food and Drug Safety (MFDS) announced this on the notifications of Orphan Drug Designation (ODD). Niktimvo (axatilimab)'s basis for ODD indication is for the treatment of 'adults and pediatric patients over 40 kg with chronic graft-versus-host disease (cGVHD) after failure of two or more systemic therapy.' In August last year, Niktimvo secured the U.S. Food and Drug Administration (FDA) approval It is a cGVHD treatment jointly developed by Incyte Corporation and Syndax Pharmaceuticals, a company in Massachusetts specializing in developing anticancer agents, through the exclusive agreement for global joint-development‧launching. GvHD is a serious complication that often occurs after allogeneic hematopoietic stem cell transplant (allo-SCT). Donor T cells from the transplanted graft recognize the recipient’s normal cells as foreign and attack them, affecting multiple organs, including the skin, gastrointestinal tract, liver, and lungs. Since symptoms can manifest throughout the body, GvHD causes additional challenges for patients who have survived allo-SCT, significantly impacting their quality of life. Corticosteroids are used as the first-line treatment; however, approximately 50% of patients fail to respond. In such cases, with no established standard therapy available, there has been an unmet need for effective treatment options. Regarding this, Niktimvo has emerged as a promising new therapeutic option with a novel mechanism of action to address the severe complications in chronic GVHD patients after previous treatments. In South Korea, Novartis Korea's 'Jakavi (ruxolitinib)' was listed on the reimbursement list in November 2023. Sanfi Korea's 'Rezurock (belumosudi)' is in the reimbursement process. The efficacy of Niktimvo was demonstrated through the AGAVE-201 study, which enrolled 241 children and adult patients with refractory and chronic GvHD who received two prior systemic therapies. Clinical results showed that the primary endpoint was met in every cohort treated with Niktimvo, with sustained responses observed across all organ systems and patient subgroups. Among patients receiving the approved dose of 0.3 mg/kg every two weeks, 75% achieved an objective response (ORR) within the first six months of treatment, with a median time to response of 1.5 months. Additionally, 60% of patients maintained their response after 12months of treatment.
- Policy
- COVID-19 Vaccine Damage Compensation Act passes NA
- by Lee, Jeong-Hwan Apr 04, 2025 05:58am
- A special law that compensates and supports patients who have suffered damage after COVID-19 vaccination was passed at the plenary session of the National Assembly on the afternoon of the 2nd. Also, a partial amendment to the Framework Act on Health and Medical Services, which includes the establishment of a Health and Medical Manforce Planning Estimation Committee under the direct control of the Minister of Health and Welfare, passed the plenary session along with the special act. The National Assembly passed the 'Special Act on Compensation for Damage Caused by COVID-19 Vaccination' with 263 in favor and 2 abstentions out of 265 members present. The Act on the Medical Manpower Planning Committee was passed at the plenary session with 247 in favor, 11 against, and 8 abstentions out of 266 members present. The Special Act expands the scope of vaccine damage compensation, such as by presuming that there is a causal relationship if the temporal correlation between COVID-19 vaccination and the disease occurrence is proven. The government has been administering vaccinations and providing state compensation in accordance with the current Infectious Disease Control and Prevention Act. However, the need for the Special Act was raised as there were indications that the damage caused by COVID-19 vaccination was not being properly compensated, due to limited causality of the damage being accepted. The Special Act, which passed the plenary session, was passed by the National Assembly Health and Welfare Committee in January with consensus from the ruling and opposition parties. The Medical Manpower Planning Committee Act, which will deliberate the number of medical school enrollees from 2027, has also passed the National Assembly, but it is unlikely to serve as a clue to resolve the conflict between the government and the medical community in Korea. The amendment proposes the establishment of the Medical Manpower Planning Committee, an independent deliberation body under the direct control of the MOHW, to deliberate on the estimation of medical personnel by job type. The committee members will consist of no more than 15 experts recommended by medical provider representative organizations, consumer representative organizations, and relevant academic societies, with the majority of the members recommended by providers, such as the Korean Medical Association (KMA), and the chairman of the committee will be elected from among the members recommended by the academic community. In addition, the revised act ensures the independence of the committee and ensures transparency by disclosing the minutes and reference materials, and the appointment of a Medical Manpower Planning center to ensure the professionalism of the planning work. When the committee estimates the size of the medical personnel required, the Health and Medical Services Policy Deliberation Committee, chaired by the Minister of MOHW, will then determine the number of medical school students.
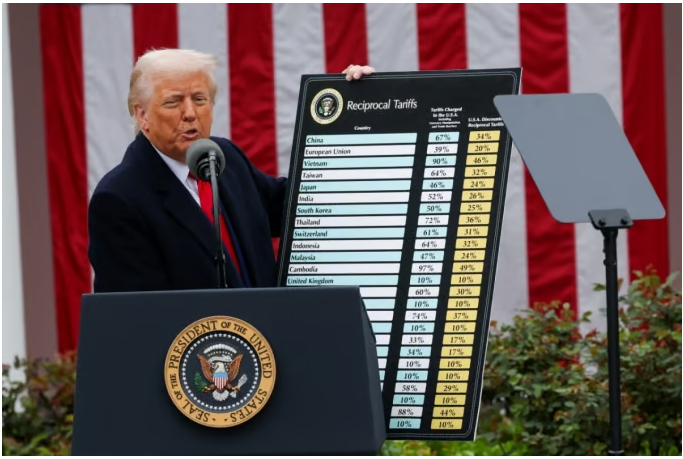
- Company
- US postpones 'Drug Tariffs', but industry eyes the situation
- by Kim, Jin-Gu Apr 04, 2025 05:57am
- The US Trump administration announced reciprocal tariffs against the entire world, but “postponed” the application of tariffs on pharmaceuticals, because it is “considering separate industry-specific tariffs” on semiconductors, key minerals, and pharmaceuticals. The domestic pharmaceutical and bio industry is relieved for now but is keeping a close eye on the White House's next move. As the U.S. has been the largest exporter of pharmaceuticals for 3 consecutive years, the specific tariff rates and items subject to the tariffs are expected to have a significant impact on the profits and losses of companies. US President Donald Trump announced on the 2nd (local time) that he would impose a 25% reciprocal tariff on South Korea. However, pharmaceuticals were excluded from this measure. The White House explained that it would impose separate tariffs on pharmaceuticals. The White House said in a separate briefing after President Trump's announcement of tariffs that “this measure does not apply to items already subject to tariffs such as automobiles, steel, aluminum, and lumber under Section 232 of the Trade Expansion Act.” It went on to explain that “President Trump is also considering separate industry-specific tariffs on semiconductors, pharmaceuticals, and key minerals, so these items are also not included in the reciprocal tariffs.” The domestic pharmaceutical and biopharmaceutical industry expressed relief for the time being because the companies have bought time to respond to the US drug tariff, at least temporarily. There are various ways for the pharmaceutical and biopharmaceutical industry to respond to the US drug tariff. In the short term, the method of moving stockpiles to the US in advance can be used. In the mid-to-long term, the industry is also considering producing products directly in the US or outsourcing manufacturing to US companies. Celltrion has secured sufficient inventories of biosimilars that can be procured locally until the third quarter of 2025. In addition, if tariffs are actually applied, the company plans to focus on exporting APIs, which are subject to lower tariffs, rather than finished drugs, which are subject to relatively higher tariffs. Furthermore, in the mid-to-long term, the company is also considering securing manufacturing facilities in the United States. SK Biopharmaceuticals, which sells the new epilepsy drug ‘Xcopri (cenobamate)’ in the United States, has a structure in which it manufactures active pharmaceutical ingredients in Korea, packs them in Canada, and then exports them to the United States. SK Biopharmaceuticals has been promoting this local production strategy in the United States for several years to stabilize its supply chain, and after transferring production technology and completing the verification procedures, it received approval from the US FDA in the second half of last year. Samsung Bioepis, which exports biosimilars to the US, is already cooperating with several contract manufacturing organizations (CMOs) overseas, so it is expected to be able to quickly adjust its strategy if a tariff is imposed. However, as the possibility of imposing tariffs on pharmaceuticals remains high, the pharmaceutical and biopharmaceutical industry is paying close attention to the specific tariff rates for pharmaceuticals and the items subject to tariffs. In this regard, President Trump announced at a press conference on the 18th of last month that the tariff on pharmaceuticals would be “25% or higher.” Some predict that basic and essential drugs will be exempted from tariffs. They note that public opinion in the United States is negative about imposing tariffs on basic and essential drugs. In the case of Huons, it exports lidocaine injections to the United States. This product is a basic drug that is in short supply in the United States and is said to have only one or two local manufacturers. So even if tariffs are imposed on pharmaceuticals, such basic drugs are expected to not be subject to them. The same is true for GC Biopharma. GC Biopharma exports the blood product Alyglo to the United States. Blood products are essential medicines in the United States, and there has been a shortage of supply in the country for several years. The company expects that blood products, including Alyglo, will not be subject to tariffs. In the United States, there is ongoing criticism that tariffs should not be imposed on basic and essential medicines. The Association for Accessible Medicines (AAM), a US generic drug lobby group, has expressed concern that if tariffs are imposed on medicines, the number of medicines in short supply, currently at 127, will surge to 215. The Kaiser Family Foundation, a US non-profit health organization, has warned that imposing tariffs on pharmaceuticals could cause the US healthcare spending growth rate to jump from the current 5% to as much as 60%. The United States has been Korea's largest pharmaceutical export destination for 3 consecutive years since 2022. Last year, pharmaceuticals exported from Korea to the United States amounted to USD 1.35809 billion (about KRW 2 trillion). This is an 18% increase compared to USD 903.3 million in 2023. However, the pharmaceutical trade balance with the United States has been in the red. The trade deficit with the United States was USD 1.1317 billion in 2022, USD 955.54 million in 2023, and USD 292.07 million last year.