- LOGIN
- MemberShip
- 2025-12-21 05:02:30
- Policy
- Polivy granted partial reimbursement after 5 years
- by Lee, Tak-Sun Jun 30, 2025 06:05am
- Roche’s Polivy (polatuzumab vedotin), a treatment for diffuse large B-cell lymphoma (DLBCL) that is currently non-reimbursed in Korea, has been added to the reimbursement list as a part of combination therapy. With the listing, the other drugs used in the combination, excluding Polivy, will be reimbursed. This measure is in accordance with the partial reimbursement policy for combination cancer therapies that was implemented in May. On the 26th, the Health Insurance Review and Assessment Service announced the reimbursement criteria for anticancer drugs and announced 2 new partial reimbursements for combination anticancer therapies that included Polivy. Partial reimbursement for combination therapy using anticancer drugs grants reimbursement to already covered drugs that are included as part of combination therapy. Previously, combination therapies were not covered by reimbursement unless they were officially added as a whole to the reimbursement criteria. The Ministry of Health and Welfare implemented this policy in May, and earlier this month, HIRA announced 35 therapies to clarify the general principles. HIRA plans to review applications for partial reimbursement for combination therapy submitted by academic societies and make additional revisions as necessary through its Cancer Disease Review Committee. As a result, two combination therapies will be added to the partial reimbursement list in July. Polivy is the first first-line treatment for DLBLC in 20 years, but it has faced difficulties in obtaining reimbursement since its approval in 2020. As a result, it remains non-reimbursed to date. The combination therapy included in the partial reimbursement criteria this time is " polatuzumab Vedotin(non-reimbursed)+rituximab, cyclophosphamide, doxorubicin, and prednisone/prednisolone (R-CHP)" for treatment-naïve adult patients with Diffuse Large B-Cell Lymphoma (DLBCL). HIRA plans to apply reimbursement for all drugs except Polivy. A combination therapy for cervical cancer that uses the immuno-oncology drug Keytruda (pembrolizumab) has also been added. As with the previous case, partial reimbursement will be provided, so all other drugs will be reimbursed excluding Keytruda and Avastin (bevacizumab). The treatments granted partial reimbursement are: pembrolizumab + paclitaxel + carboplatin ± bevacizumab and pembrolizumab + paclitaxel + cisplatin ± bevacizumab. Reimbursement will be applied to patients with recurrent, metastatic (stage IV) cervical cancer or stage IB2 or higher that meet one or more of the following conditions: ▲positive pelvic lymph nodes (pelvic LN) after surgery, ▲positive para-aortic lymph nodes (para-aortic LN) after surgery, or ▲positive parametrium after surgery (stage I or higher, palliative treatment). The reimbursement criteria also include revisions to Tier 1 and 2 anticancer drugs. HIRA explained, “When the criteria were first established in 2006, in the details regarding the application criteria and methods for reimbursement of drugs prescribed and administered to cancer patients, drugs subject to re-evaluation, orphan drugs, or drugs with potential for abuse were classified as Tier 2 drugs and was granted use within the scope of its reimbursement criteria. Tier 1 drugs were to be administered at the discretion of the doctors within the scope of the indication and general principles for anticancer therapy." "However, since the initial enactment, new clinical evidence is being updated every year due to drug price fluctuations, the listing of generic drugs and many new high-priced anticancer drugs, and the development of new drugs with new mechanisms of actions, rendering it necessary to review the old treatment guidelines (mainly the deletion of Tier 1 anticancer drugs that are highly toxic and ineffective and the reclassification of Tier 2 anticancer drugs) and recommend the use of anticancer treatments that are more clinically proven and safer for the public. In this regard, we gathered opinions from academic societies and held TFT meetings on improving the reimbursement criteria for anticancer drugs to establish a reimbursement criteria (draft) for anticancer drugs. After final discussions by the Cancer Disease Deliberation Committee, we established new reimbursement criteria for anticancer therapies that removed the classification of anticancer drugs into Tiers 1 and 2.” However still, for cancers that are difficult to establish clinical evidence due to the small number of drug options and patient population, the current system will be maintained. Detailed information on the revisions can be found on the HIRA website (System/Policy → Drug Criteria Information → Drugs and Therapies Used for Cancer).
- Opinion
- [Reporter's View] For local gov't seeking biotech diplomacy
- by Whang, byung-woo Jun 30, 2025 06:05am
- 'BIO USA,' held in Boston, U.S., is the world's largest biotech industry conference. Notably, this year's conference was attended by officials from Korea's local government. The attending officials aimed to promote local biotech clusters and seek potential investment partners. Through this event, they intended to establish a gamer-changer to their regional growth. However, some industry officials showed both anticipation and concern. Korean local governments attending this year's BIO USA strived to meditate on biotech diplomacy. For instance, Nowon District of Seoul hosted a press conference near the event location and presented plans for 'Seoul-Digital Bio City (S-DBC).' Currently, Nowon District is reportedly discussing investment opportunities with two biotech companies in South Korea to build a biotech cluster in Chong-dong Training Depot. Daejeon City, as a key bio-cluster holding local government, also sought technology transfer and joint research partnerships through MOU signings with overseas research institutions and IR events targeting investors. Furthermore, Siheung City, Gyeonggi Province, which is promoting a biotech-cluster through an investment agreement of approximately KRW 2.2 trillion with Chong Kun Dang, also visited the event. Although the enthusiasm of these local governments was evident, accomplishments have not yet been achieved. Some local governments have only just begun their steps into the pharmaceutical and bio-industry, so news of significant investment attraction or contract signings is scarce. An industry official pointed out, "While local government's interest and investment in the bio-industry are welcome. However, if the focus is solely on trying without deliveries, it will ultimately be judged as mere showmanship." Ultimately, if local governments' overseas efforts do not lead to effective investment exchanges, they may not yield tangible results despite significant time and budget expenditure. While various local governments view the pharmaceutical and biotech industries as a future growth engine and actively engage with them, not all have a favorable view. This is partly because there are precedents of bio-clusters located across the country. Currently, there are between 20-30 large and small bio-specialized complexes established in Korea, but questions remain about whether all these clusters are functioning. Recently, the focus has shifted to improving efficiency and enhancing the competitiveness of existing bio-clusters instead of merely creating new ones. Local governments also view that it's necessary to clarify their position and strengths within this larger picture, and strive to build complementary ecosystems by avoiding redundant investment or competition. The choice of local governments to invest in the biotech industry, seeing it as a key opportunity for job creation, is not necessarily deserving of criticism. However, there are negative view regarding the aspirations of some local governments to attract global big pharma's offices and R&D centers. A biotech industry official advised, "Ultimately, to attract global big pharma, clusters in South Korea must grow to a level that makes them desirable. It's not a problem that can be solved by local governments merely providing buildings and land. They must create a self-sustaining ecosystem where excellent research personnel and companies gather." Ultimately, proper direction and cooperation are needed. For the local government's efforts in Boston to yield tangible results rather than empty echoes, industry stakeholders and policymakers need to work as a unified team, drawing a larger picture, rather than pursuing individual goals, self-serving efforts by the central government, local governments, and private companies.
- Company
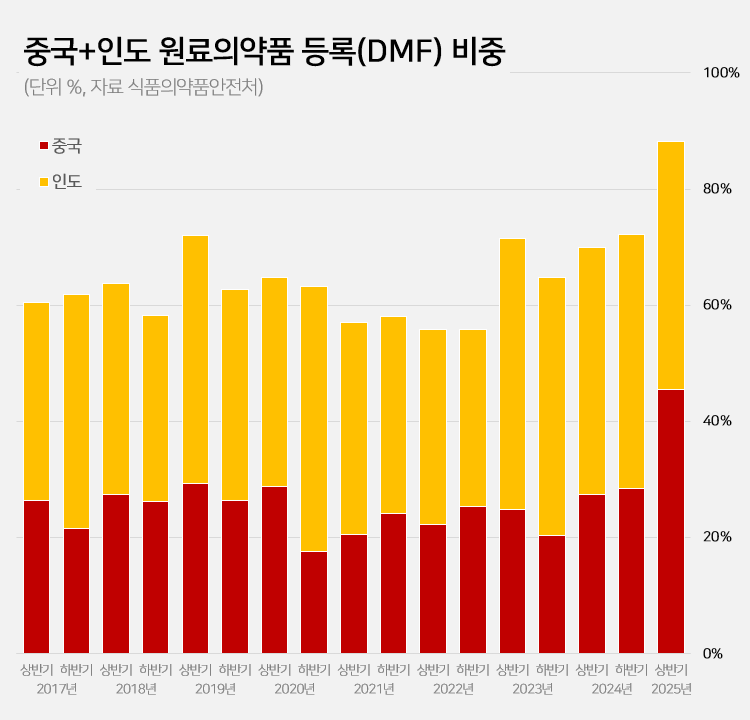
- 88% registered APIs imported from China or India
- by Kim, Jin-Gu Jun 27, 2025 06:04am
- Amid a surge in the number of drug master file registrations in the first half of this year, the share of raw materials from China and India rose to 88.2%. This is a sharp increase compared to the average of 62.1% share the two countries had during the past 5 years. This is attributed to the large number of previously delayed raw material drug registrations that had been made upon the relaxation of DMF regulations, as well as the domestic pharmaceutical and bio industry's increased use of raw materials from China and India to reduce costs. 606 DMF registration from China and India source materials made in the first half of this year... accounts for 88%, which is the highest-ever share According to the Ministry of Food and Drug Safety on the 26th, 687 DMF registrations were made in the first half of this year. Among them, 313 raw materials were from China and 293 were from India. The two countries combined accounted for 606 cases or 88.2% of the total DMF registrations. This is the highest proportion ever recorded for a half-year period. Until last year, the proportion of Chinese and Indian raw materials had never exceeded 75%. The average proportion of Chinese and Indian DMF imports over the past 5 years was 62.1%, which is more than 26 percentage points higher than in the first half of this year. The proportion of Chinese and Indian imports in the DMF has increased rapidly over the past 3 years. After steadily declining since the first half of 2019, the share of Chinese and Indian DMF rose to 55.8% in the first half of 2022 and then began to increase. It reached 69.9% in the first half of last year and 72.2% in the second half. In the first half of this year, it soared to nearly 90%. Pharmaceutical industry's dependence on Chinese and Indian raw materials deepens amid cost pressures The rapid increase in Chinese and Indian DMF is attributed to cost reduction pressures in the pharmaceutical and bio industry. China and India are representative “low-cost mass production bases” in the global raw material drug market. Following the global economic downturn after the pandemic, the pharmaceutical and bio industry in general faced a decline in profitability. As a result, attempts to reduce costs were made, which led to an increase in the use of Chinese and Indian DMF. An industry insider explained, “Domestic pharmaceutical and biotechnology companies are feeling a significant burden from manufacturing costs due to high exchange rates, rising labor costs, and declining profitability. Chinese and Indian raw materials are sometimes almost half the price of domestically produced materials, leading to increased use of imported materials.” Additionally, the relaxation of DMF regulations has further increased the use of Chinese and Indian raw materials. The government eased DMF requirements earlier this year by replacing on-site GMP inspections with the submission of GMP certificates and reducing the administrative processing period from 120 days to 20 days. As a result, imported raw materials for which registration had been delayed were registered en masse. In particular, it is analyzed that the abolition of on-site inspections has led to a significant increase in Chinese and Indian raw materials. In the past, inspections in these two countries were physically difficult, and administrative procedures complex, often causing delays in registration. This year, however, registration became possible with only a GMP certificate, significantly lowering barriers, and leading to a significant increase in the registration of Chinese and Indian raw materials. Domestic raw material share in DMF only 5%, raising concerns about increased dependence on Chinese and Indian products On the other hand, the share of domestically produced raw materials registered in the DMF has decreased significantly. In the first half of this year, the share of domestically produced raw materials registered was only 4.9% (34 cases). This is less than half of the 12.6% recorded in the second half of last year. The share of DMF registrations of raw materials from Europe and Asia also decreased sharply. The share of European raw materials decreased by more than 10 percentage points from 14.5% in the first half of last year to 4.4% in the first half of this year. The number also decreased from 37 to 30. The share of DMF registrations of raw materials from Asian countries other than China and India also decreased from 3.9% to 1.5%. Concerns have been raised that the dependence on raw materials from China and India may become excessively high. If this trend intensifies, it could pose a threat to the stability of domestic drug supply. In fact, during the early stages of the COVID-19 pandemic, export restrictions imposed by China and India directly impacted domestic drug production. A pharmaceutical industry insider stated, “If raw material production becomes overly concentrated in specific countries, it becomes vulnerable to external factors such as export restrictions, logistics disruptions, and sharp exchange rate fluctuations. In the long term, policies are needed to strengthen domestic raw material production capabilities and provide various incentives for the use of domestically produced raw materials.”
- Policy
- Ensuring stable supply of drugs in short supply
- by Lee, Jeong-Hwan Jun 27, 2025 06:03am
- Following President Lee Jae-myung's pledge to establish a stable supply system for drugs with supply shortages, attention is drawn to the Ministry of Health and Welfare's (MOHW) opinion that a social consensus on the criteria and scope of 'supply shortage' is first needed. It is anticipated that legislative review in the National Assembly to resolve the issue of drug shortages will progress if criteria for drug shortages are established, including whether the causes of the shortages are isolated or chronic. On June 26, an MOHW official met with the Korea Special Press Association and explained, "The government also agrees on the need to reform national governance to respond to drugs with supply shortages." The official proposed the necessity of establishing a definition and criteria for drugs with supply shortages to find solutions to the drug shortage problem properly. Establishing the criteria first is necessary because drug shortage issues arise from various types and cases, and finding a consensus on how to quantify them is essential for laying the groundwork to establish policies and proceed with legislation. The MOHW stated that while national essential medicines are designated through various criteria and procedures, defining criteria for drugs with supply shortages will be challenging due to their often variable nature, such as differing shortage durations for each case. An MOHW official explained, "It's crucial how we measure supply shortages. We monitor the situation through supply history reports, but it's difficult to confirm why shortages occur at the final distribution stage," and added, "Since we do not have any information on the inventory levels so there are data limitations in determining whether a shortage is due to an absolute lack of supply or a hoarding problem." The official further explained, "It takes about 2-3 months for data on actual drug prescriptions and usage to accumulate. Hospitals and pharmacies don't report every time a drug is used, and it's even harder to secure data for non-reimbursable drugs," and added, "We need to establish some level of supply shortage criteria to come up with countermeasures. Even during public-private meetings, we discussed whether a drug with supply shortage needed an intervention or not." Meanwhile, four amendments to the Pharmaceutical Affairs Act aimed at resolving the issue of drugs with supply shortages are currently pending in the National Assembly (proposed by Rep. Han Jeoung-ae, Rep. Kim Yoon, Rep. Kim Sunmin, and Rep. Seo Mi-hwa).
- Company
- Tevimbra adds esophageal, gastric, lung cancer indications
- by Whang, byung-woo Jun 27, 2025 06:02am
- Pic of TevimbraBeiGene Korea (Name to be changed to BeOne Medicine Korea) announced that its immuno-oncology drug Tevimbra (tislelizumab) has been approved by the Ministry of Food and Drug Safety for additional indications for esophageal cancer, gastric cancer, and non-small cell lung cancer. With the additional approval, Tembriva can now be used as a first- or second-line treatment for a total of 5 indications across 3 solid tumor types. The approved cancer types are: esophageal squamous cell carcinoma (ESCC), gastric or gastroesophageal junction adenocarcinoma (G/GEJ), and non-small cell lung cancer (NSCLC). Tevimbra demonstrated efficacy and safety for the indications in the RATIONALE clinical trial series (RATIONALE-303, 304, 305, 306, 307), which served as the basis for this approval. In particular, the drugs’ clinical benefits were observed in the overall patient population for esophageal squamous cell carcinoma and gastric or gastroesophageal junction adenocarcinoma and showed consistent results in pre-specified subgroups based on PD-L1 expression levels. Such benefits of Tevimbra were also reflected in global treatment guidelines and is recommended at a high level by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO). Tevimbra employs a dual mechanism of action that effectively blocks PD-L1 while minimizing binding to Fc-gamma receptors (FcγR), thereby inducing potent antitumor responses through a mechanism distinct from that of existing immuno-oncology agents. Also, the drug demonstrated superior PD-1/PD-L1 blocking efficacy (>99%) compared to other immunotherapy agents of the same class, and according to the company, it has a higher binding affinity and a half-life 30-80 times longer than existing drugs, suggesting a more sustained therapeutic effect. In addition, by minimizing binding to the Fc gamma (Fcγ) receptor of the antibody, the drug enhanced the sustainability and stability of the immune response. Ji-Hye Yang, General Manager of BeOne Medicines Korea, explained, “Based on its differentiated mechanism and long-term clinical data, Tevimbra is gaining attention as a new standard of care that surpasses the limitations of existing immunotherapy drugs by offering treatment performance comparable to global benchmarks, as well as treatment sustainability and financial predictability.” Yang added, “We are particularly pleased to offer new treatment opportunities for underserved patients. in the first-line setting for esophageal squamous cell carcinoma, as Tevimbra is the only approved immunotherapy in Korea that can be used regardless of PD-L1 expression levels.” Meanwhile, BeiGene changed its corporate name to BeOne Medicines under the vision of “Overcoming Cancer Together” and is accelerating the expansion of its next-generation anti-cancer portfolio, including ADC (antibody-drug conjugates) and protein degraders, with Switzerland as its global hub. The domestic subsidiary will also complete its rebranding as ‘BeOne Medicines Korea’ by June 30 and plans to accelerate its growth in the solid tumor and blood cancer treatment markets based on its 2 products and 11 indications.
- Company
- KPTA ‘KOR-CHN-JPN supply cooperation to bring $12B effect'
- by Kim, Jin-Gu Jun 27, 2025 06:02am
- The Korea Pharmaceutical Traders Association (KPTA), China Chamber of Commerce for Import & Export of Medicines & Health Products (CCMPHIE), and Japan Pharmaceutical Traders Association (JPTA) announced on June 25 that they signed a memorandum of understanding (MOU) for the stabilization of the pharmaceutical supply chain at the Korea Pavilion in the CPHI & PMEC CHINA exhibition hall in Shanghai, China. The signing ceremony was attended by Hyung-seon Ryu, Chairman of the KPTA; Zhou Hui, President of CCCMHPIE; Ichiro Fujikawa, President of JPTA; Young-soo Jeong, Director of the KOTRA Shanghai Trade Office; and over 20 representatives and officials from each country. The MOU was signed to improve the global pharmaceutical supply chain, which has become unstable following the COVID-19 pandemic, and to enhance the three countries' capability to respond to health crises through cooperation. The three associations play leading roles in the pharmaceutical trade and distribution sectors. Through the MOU, the parties agreed to collaborate on ▲ the export, import, development, and supply of essential and active pharmaceutical ingredients; ▲ exchange of pharmaceutical research personnel, technology, and information; and ▲ joint hosting of seminars, academic conferences, and workshops to promote pharmaceutical trade. They plan to concretize the outcomes of the MOU through the implementation of practical joint projects. The KPTA stated that if supply chain stabilization is realized through this agreement, it will bring an annual economic effect of approximately USD 12 billion through ▲the reduction of raw material inventory costs ▲reduction of procurement costs through joint purchasing ▲reduction of health crisis response costs ▲trade creation effects from the activation of pharmaceutical trade among the three countries ▲trade diversion effects from replacing overseas pharmaceutical imports with intra-regional trade. Also, based on this agreement, it is anticipated that a three-country contract manufacturing (CDMO) model may be forged, enabling Korean companies to produce pharmaceuticals at competitive prices in China, register them in Japan, and enter the European market. Cases where domestic pharmaceutical companies experienced disruptions in imported raw material supplies also also expected to decrease, as the partnership will allow Korean companies to secure stable supply through emergency supply contracts with Chinese and Japanese companies. Hyung-seon Ryu, Chairman of the KPTA, stated, “Korea has strengths in producing high-quality drugs, Japan in precision manufacturing technology and rare drug raw material technology, and China in large-scale production and supply capabilities. This MOU will greatly contribute to the stability of the entire Northeast Asian supply chain and the enhancement of global competitiveness. We ask Korean companies to actively participate in various areas, including follow-up technical collaboration, research and development, and contract manufacturing.” Zhou Hui, Chairman of CCCMHPIE, said, “This agreement is the result of close collaboration between the 3 countries and will contribute not only to the stable supply of essential and raw pharmaceuticals but also to the creation of new business opportunities. It will serve as a leading model for cooperation in the East Asian pharmaceutical industry.” Ichiro Fujikawa, President of the JPTA, commented, “Korea and China are Japan’s core partners for raw materials and finished pharmaceutical products. This agreement will help alleviate the shortage of pharmaceutical supplies in Northeast Asia and positively impact the growth and development of each country’s pharmaceutical industry.”
- Company
- ‘Wegovy, a game-changer for high-risk obesity patients’
- by Whang, byung-woo Jun 26, 2025 06:08am
- Obesity is a cause of various metabolic syndromes and a major risk factor for cardiovascular disease. In fact, approximately 80% of patients hospitalized for cardiovascular disease are obese, and studies have shown that the risk of cardiovascular events in obese patients is up to twice as high as in those of normal weight. Over the past 20 years, the mortality rate from obesity-related cardiovascular diseases has increased significantly, with approximately two-thirds of obesity-related deaths attributed to cardiovascular diseases. Recently, semaglutide (brand name: Wegovy), a GLP-1 receptor agonist, has opened a new treatment paradigm in obesity treatment, demonstrating efficacy in reducing the risk of major cardiovascular events in high-risk obese patients. Kim Kyung-hee, professor of cardiology at Incheon Sejong Hospital (Director of the Heart Transplant Center), met with Dailypharm and emphasized the importance of obesity treatment for the prevention of cardiovascular disease. Obesity increases the risk of early onset of cardiovascular disease... “The number of young patients is also on the rise” Obesity is a precursor to various metabolic diseases, and inflammatory substances secreted from visceral fat reduce blood vessel elasticity. According to Professor Kim, these changes lead to hypertension, diabetes, and hyperlipidemia, which in turn greatly increase the risk of early onset of coronary artery disease and heart failure even in younger age groups. Kyung-hee Kim, Professor of Cardiology, Incheon Sejong Hospital (Director of the Heart Transplant Center) Professor Kim said, “Obesity can be a cause of all cardiovascular diseases, and there is a recent trend of an increase in patients with high blood pressure or symptoms of heart failure from a young age. Recently, the prognosis of severely obese patients is generally worse than that of the general population, but even in lean individuals, and those with sufficient muscle mass tend to have a better prognosis.” Kim further explained, “In cases of cardiovascular diseases such as angina or heart failure, there is a tendency for weight loss and reduced muscle mass due to decreased appetite and nutrient intake. Therefore, obesity typically occurs first, followed by cardiovascular diseases in most cases.” In other words, obesity often acts as a precursor to cardiovascular disease. In this regard, semaglutide is regarded a game changer in the fields of obesity treatment and cardiovascular disease prevention. Semaglutide was approved by the Ministry of Food and Drug Safety in April 2024 as an anti-obesity treatment for patients with a BMI of 27 kg/m² or higher (with comorbidities) or 30 kg/m² or higher, and in July of the same year, it was additionally approved for reducing the risk of cardiovascular events in overweight and obese adult patients with confirmed cardiovascular disease. Professor Kim emphasized the clinical value of semaglutide not merely as a weight-loss aid but as a preventive therapy for cardiovascular disease. In particular, KIM highlighted findings from the SELECT trial, a pivotal clinical study on semaglutide, where the drug demonstrated an additional 20% reduction in the risk of major adverse cardiovascular events (MACE) when added to standard care in patients already receiving conventional treatments. Professor Kim explained, “In the SELECT study, approximately 90% of participants were already receiving standard treatment, but when they added semaglutide, an additional 20% reduction in MACE risk was observed. This result demonstrates that semaglutide can make a substantial contribution to improving outcomes in high-risk patient populations where existing treatments have limitations.” According to the detailed results of the SELECT trial, over an average follow-up period of approximately 3.3 years, the semaglutide 2.4 mg group showed a statistically significant 20% reduction in the risk of cardiovascular death, nonfatal myocardial infarction, or stroke compared to the placebo group. Professor Kim added, “Semaglutide regulates the appetite center in the brain, delays gastric emptying to induce weight loss, and further improves cardiovascular risk factors through its anti-inflammatory effects. While weight loss may also play a role, we believe that the drug’s anti-inflammatory effect plays a very significant role in cardiovascular health.” In particular, Professor Kim emphasized that semaglutide is not simply a weight loss drug, but a scientifically proven cardiovascular treatment option. He said, “Semaglutide is a must-use drug for patients who are severely obese or have a BMI of 27 kg/m² or higher with cardiovascular disease.” “Limitations remain on its reimbursement... Selective reimbursement support needed for high-risk obese patients” Although semaglutide has emerged as an important drug that should be considered as part of a standard treatment for obese patients at high risk of cardiovascular disease, there are currently practical limitations to its access in Korea. This is because it is not yet covered by insurance in Korea. Professor Kim said, “Drugs such as semaglutide carry a certain risk of misuse, so caution should be exercised when expanding reimbursement to all patient groups. However, I believe it is desirable to allow reimbursement through strict criteria and screening procedures for high-risk groups, such as patients with severe obesity and cardiovascular complications, for whom clear therapeutic effects can be expected.” In fact, limiting reimbursement to groups with a clear clinical need for reimbursement, such as patients with a body mass index (BMI) of 27 kg/m² or higher and obesity-related complications or cardiovascular disease, may be a realistic alternative. Professor Kim also predicted that discussions on whether to continue reimbursement will be necessary when semaglutide significantly improves a patient’s BMI. He said, "If patients with a BMI of 30 kg/m² or higher are administered semaglutide and their BMI falls below 26 kg/m² due to weight loss, it may be possible to consider limiting the reimbursement period to the initial 4-6 months. However, since there is currently insufficient long-term data in Korea and some patients experience weight regain after 6 months of treatment based on clinical experience, further review of long-term management strategies is necessary.” To address current issues surrounding reimbursement coverage and costs, Professor Kim is currently conducting an economic evaluation study. “We are analyzing how much the number of medications taken by patients can be reduced when they lose weight after 6 months or a year through bariatric surgery or semaglutide treatment, and we expect this to be significant in terms of establishing future treatment strategies and fiscal efficiency.” Ultimately, Professor Kim believes that obesity treatment should not end with medication alone but must include comprehensive management to help patients fundamentally improve their lifestyle habits. Professor Kim emphasized, “Patients with a BMI of 30 kg/m² or higher often find exercise difficult, so they should be actively educated to combine medication with walking exercises, maintain a high-quality diet, reduce carbohydrate intake, and abstain from alcohol and smoking.” “Early intervention in obesity treatment is necessary to maximize preventive effects” Professional counseling and lifestyle education support are essential to increase the effectiveness of obesity treatment. However, the reality of how difficult it is to provide sufficient counseling in the current outpatient setting is also pointed out as an issue. Professor Kim said, “It is difficult to check blood pressure, assess the patient's condition, perform a physical examination, and explain lifestyle correction measures within the 5 minutes of consultation time allocated per patient. At least 7-10 minutes are necessary for proper treatment.” In this regard, Professor Kim proposed the establishment of a lifestyle education program and a new fee schedule to hire dedicated personnel to overcome such limitations. He explained, “Under the current system, separate reimbursement rates for education provided by specialized nurses need to be introduced, and institutional and financial support would also be needed to manage these personnel. Overall, I believe that establishing an environment where lifestyle education can be systematically implemented is essential to improving the quality of care for obese patients. In particular, Professor Kim emphasized, “Patients with hypertension are at high risk of developing heart failure over time. Starting medication early and educating young obese patients with hypertension on proper lifestyle habits can prevent serious complications and repeated hospitalizations.” In other words, Kim believes preventive treatment and lifestyle improvement efforts targeting young obese patients are expected to lead to macro-level medical cost savings in the future. Finally, Professor Kim emphasized that “Patients should always be treated with scientifically validated medications first. Drug treatment alone is not sufficient and must be accompanied by lifestyle modifications and proper dietary management.”
- Company
- The 2nd KRAS-targeted cancer drug 'Krazati' expected
- by Eo, Yun-Ho Jun 26, 2025 06:08am
- Product photo of Krazati The second KRAS inhibitor is expected to be commercialized in South Korea. Bristol Myers Squibb (BMS) Korea recently submitted a marketing authorization application to the Ministry of Food and Drug Safety (MFDS) for its anti-cancer drug, Krazati (adagrasib). Krazati was also designated as an orphan drug in January. It is indicated for the treatment of 'locally advanced or metastatic non-small cell lung cancer (NSCLC) with a KRAS G12C mutation, previously treated with at least one prior therapy.' Krazati received accelerated approval from the U.S. FDA in December 2022. It is the second KRAS inhibitor receiving approval, following Amgen's 'Lumakras (sotorasib),' which was approved in 2021. The development of KRAS-targeted anti-cancer drugs has come approximately 40 years after the initial discovery of the oncogene. Amgen and BMS are engaged in fierce competition to dominate this new market. Lumakras and Krazati share many similarities, including their target mutation and indications. Both target the KRAS G12C mutation, and their initial approved indication is NSCLC. Both companies are also conducting clinical trials in combination with compounds with different mechanisms of action developed in-house or through collaborations. Meanwhile, Krazati's initial approval was based on cohorts from the KRYSTAL-1 study who are eligible for Phase 2 trials. Last year, the primary analysis results of the confirmatory Phase 3 study were disclosed. This study compared Krazati with docetaxel in 301 patients with previously treated KRAS G12C-mutated locally advanced or metastatic NSCLC. These patients had previously received platinum-based chemotherapy and anti-PD-1/PD-L1 immunotherapy. They were randomized 1:1 to either the Krazati treatment group or the docetaxel treatment group. The primary endpoint was progression-free survival (PFS) as assessed by blinded independent central review (BICR). After 9.4 months of follow-up, the median PFS for Krazati was 5.49 months, which met the primary endpoint by reducing the risk of disease progression or death by 42% compared to 3.84 months for the docetaxel group.
- Product
- 'Govn’t must take strong action against pharma rebates'
- by Kang, Hye-Kyung Jun 26, 2025 06:08am
- The Korean Pharmacists for Democratic Society (President Gyeong-rim Jeon, KPDS) has urged the government to take strong action against pharmaceutical company rebates. KPDS issued a statement regarding rebates made by a major domestic pharmaceutical company that JTBC reported. In the statement, KPDS said, “This is not an individual incident, but clear evidence of widespread illegal rebates rampant throughout the pharmaceutical industry. “Medicines prescribed by doctors are not ordinary commodities, but special goods that directly affect patients' lives and health. The Pharmaceutical Affairs Act and Medical Service Act fundamentally prohibit pharmaceutical companies from providing economic benefits to medical professionals.” However, according to the JTBC report, the pharmaceutical company in question blatantly violated legal restrictions. The KPDS criticized, “What is even more outrageous is the government authorities' incompetent response to these clear illegal acts. Using a shortage of personnel as an excuse to avoid addressing the issue raises suspicions that there may be an intent to cover up the incident.” They went on to say, “The explanation that this was a legitimate new drug promotion activity is a clear lie, and the government must immediately implement strong measures to eradicate illegal rebates.” In other words, the KPDS believes the MOHW and the police must reinvestigate the case and severely punish those involved. The KPDS proposed the following measures: ▲reinvestigation through the formation of a special investigation team; ▲a comprehensive investigation by the MOHW into the provision of financial benefits such as support for academic conferences, lecture fees, and consulting fees; and ▲strong administrative measures such as drug price reductions when illegal rebates are detected. They emphasized, “We can no longer tolerate the illegal rebate cartel that threatens the health insurance budget and public health. The government must use this incident as an opportunity to demonstrate its firm commitment to eradicating illegal rebates in the pharmaceutical industry.”
- Policy
- Yungjin, Ildong’s Ofev generics enter market at half price
- by Lee, Tak-Sun Jun 26, 2025 06:07am
- A generic version of Ofev (nintedanib), a treatment for chronic fibrotic interstitial lung disease), will enter the market at half the price of the original drug. With the entry of generic drugs, the Ofev market now faces competition, just two months after the original drug was listed for reimbursement. According to industry sources on the 25th, Yungjin and Ildong Pharmacuetical will list the generic version of Ofev with reimbursement at a price much lower than the calculated price, differentiating it from the original product. Also, the original product is a capsule formulation, while the generic version is a tablet formulation. When reimbursement was approved in May, the maximum insurance price for Boehringer Ingelheim Korea's Ofev Soft Capsules 100 mg was set at KRW 29,600, and Ofev Soft Capsules 150 mg at KRW 26,220. The generic versions are much cheaper than this. Three generic drugs will be listed for reimbursement next month: Yungjin Pharmaceutical's Nintebro Tab 150 mg and Nintebro 100 Tab mg, and Ildong Pharmaceutical's Cuninta Tab 150 mg. Among them, Nintebro 100 mg has a maximum price of KRW 9,000, less than half the original Ofev Soft Capsule 100 mg (KRW 20,960). Nintebro 150 mg is KRW 15,000, and Cuninta 150mg is KRW 13,500. When considering how Ofev Soft Cap 150mg costs KRW 26,220, the generic versions cost half the original price. Being rare disease treatments, the generic versions were eligible to receive the same price as the original Ofev, but it is believed that the companies significantly lowered the price in consideration of market competitiveness. As a result, patients will now be able to purchase the same ingredient drug at a lower price. In May, after nine years since being approved in Korea, Ofev was listed for reimbursement as a treatment for chronic fibrotic interstitial lung disease. Due to the delay in reimbursement listing, the substance patent expired on January 25. This is how generic versions were able to enter only 2 months after the original drug was listed for reimbursement. Meanwhile, Yungjin and Ildong, which are launching the generic versions, are competing fiercely in the pirfenidone market, a representative treatment for idiopathic pulmonary fibrosis, which is another indication for Ofev.