- LOGIN
- MemberShip
- 2025-12-21 05:01:29
- Policy
- Will the general diabetes criteria be revised after 14 yrs?
- by Lee, Tak-Sun Jul 16, 2025 06:09am
- The National Health Insurance Service (NHIS) is currently reviewing a comprehensive revision of the general principles for diabetes drug reimbursement criteria, which have been in place for 14 years. It is garnering significant attention from the industry. Following recent trends, significant changes are anticipated, including the removal of metformin and sulfonylurea (SU) as first-line agents and a reorganization of approved dual therapies. However, concerns about increased national health insurance expenditure make the government's ultimate decision an attention. According to industry sources, on July 14, the Health Insurance Review & Assessment Service (HIRA) is reviewing a comprehensive revision of the general principles for diabetes drug reimbursement criteria, as requested by organizations such as the Korean Association of Internal Medicine. The current general principles for diabetes drugs, which designate metformin and SU as first-line agents, were first established in 2011. At the time, the Ministry of Health and Welfare (MOHW) formulated these principles to align with prescription patterns, encourage cost-effective drug use, and reduce the financial burden on the national health insurance. However, after 14 years, the general principles for diabetes drugs are now considered outdated. Indeed, this year, the Korean Diabetes Association revised its "9th Edition Diabetes Treatment Guidelines," removing the detail that metformin is the first-line treatment for type 2 diabetes. Instead, the guidelines recommended prioritizing the use of the latest drugs based on the patient's pathophysiology and clinical characteristics. The Korean Diabetes Association explained that this revision is an evidence-based guideline with clear levels of evidence and benefits. Not only in Korea but also in developed countries like the United States, metformin is no longer recommended as a first-line agent. The Korean Diabetes Association is said to have gathered these opinions and requested a comprehensive revision from HIRA earlier this year. HIRA is reportedly reviewing the comprehensive revision proposal. The pharmaceutical industry is also requesting a comprehensive revision, arguing that the current general principles are contrary to the latest medical practices and trends. Analysis suggests that the revised principles are highly likely to include comprehensive changes to the general principles, from the status of metformin and SU as first-line treatments to the approved dual therapies. However, if SGLT-2 inhibitors, DPP-4 inhibitors, or GLP-1 analogues are recommended as first-line treatments instead of metformin or SU, there is a high possibility of a significant increase in national health insurance expenditure.Therefore, health insurance authorities are expected to review the cost-effectiveness of these measures closely. An official from the pharmaceutical industry said, "If the current general principles for diabetes drug reimbursement criteria are comprehensively revised, it will have a significant impact on the market, and pharmaceutical companies will inevitably have to consider their profit and loss," and added, "I believe the NHIS will also establish measures to minimize financial expenditure."
- Company
- Potential changes to reimb policy for new anticancer drugs
- by Moon, sung-ho Jul 16, 2025 06:08am
- Multinational pharmaceutical companies are actively pursuing to secure reimbursement for their anti-cancer drugs as first-line treatment options. Based on accumulated clinical research, companies are competitively applying for reimbursement from the government, putting in all efforts to accomplish this by the second half of this year. Recently, several argued that the current reimbursement review paradigm, which has focused on 'life extension,' should undergo a significant transformation to center around the 'improvement of quality of life.' In other words, they argue that reimbursement should be discussed primarily for first-line treatment options, rather than late-stage treatments for specific cancers, with a focus on directly improving quality of life. According to the pharmaceutical industry, on July 14, multinational pharmaceutical companies have recently applied to the Health Insurance Review & Assessment Service (HIRA) for reimbursement of their anti-cancer drugs as first-line treatment options. For instance, 'Astellas Pharma Korea' is one of them. Astellas recently re-applied for reimbursement for the Padcev (enfortumab vedotin)-Keytruda (pembrolizumab, MSD) combination therapy as a first-line treatment for urothelial carcinoma. In addition, they concurrently applied for Padcev monotherapy for second-line or later treatment. Notably, Astellas re-applied for both first-line and second-line or later treatments at once, bringing both therapies to the Cancer Disease Review Committee (CDRC) discussion table. While the monotherapy, used in second-line or latertreatment, had passed the CDRC and was undergoing cost-effectiveness evaluation, the combination therapy obtained domestic approval and rapidly gained presence in clinical settings. Therefore, the analysis suggests that Astellas resubmitted both together. In other words, both the combination therapy and monotherapy are now on the CDRC table for discussion. Dr. In-Keun Park of Asan Medical Center, Seoul (Department of Oncology), said, "Using Padcev earlier in the treatment has an advantage in terms of overall survival (OS) compared to using it later." He added, "Realistically, due to cost issues, the dosage is often adjusted (contrary to clinical standards). In some cases, patients are even reducing their body weight to lower the drug dosage due to drug costs." Dr. Park added, "In clinical practice, the proportion of patients who transition from first-line to second-line treatment, and from second-line to third-line treatment, decreases sharply," and emphasized, "Considering that first-line combination therapies are already introduced and used in Korea, discussions for reimbursement of first-line combination therapies can no longer be delayed." Furthermore, the competition for reimbursement of first-line treatments to secure market dominance in the gastric cancer treatment market in the second half of this year is accelerating. Keytruda passed the CDRC in the first half of this year and is currently undergoing cost-effectiveness evaluation at a subcommittee of the Drug Reimbursement Evaluation Committee (DREC). As the only immunotherapy that can be used not only in HER2-negative but also HER2-positive patients, Keytruda, as a first-line option in Stage 4 gastric cancer treatment, is expected to grow significantly depending on whether reimbursement is applied. Additionally, BeOne Medicines Korea (formerly BeiGene Korea) has also applied to HIRA for reimbursement for five new indications, including gastric cancer, for its immunotherapy Tevimbra (tislelizumab). This means that BeOne Medicines Korea had immediately applied for HIRA after obtaining domestic approval by the MFDS in June. Additionally, Astellas has also re-applied for reimbursement for 'Vyloy (zolbetuximab),' a Claudin 18.2 gastric cancer targeted therapy. The company has recently been promoting this drug alongside Padcev. This re-application comes approximately four months after Astellas failed to achieve reimbursement listing from the CDRC in February. An Astellas official explained, "Korean medical professionals and patients sincerely hope for rapid reimbursement of Vyloy in the treatment of HER2-negative and Claudin 18.2-positive metastatic gastric cancer, where there are no other treatment options." He added, "We have faithfully reflected HIRA's supplementary requests and completed the re-application for reimbursement. We will do our best to ensure that the best treatment option is quickly introduced to patients." As multinational pharmaceutical companies continue to aim for reimbursement of anti-cancer drugs as first-line treatment options, the government authorities will likely face an increased load of responsibility for evaluation. This is because reimbursement of initial treatments naturally leads to increased financial burden on the national health insurance. However, reimbursement cannot be denied for treatments with proven efficacy in clinical research due to the financial burden. Therefore, opinions are emerging that the government's reimbursement discussion paradigm must undergo a significant transformation. They imply that a shift in the discussion focus from 'life extension' to 'improvement of quality of life.' In fact, until now, both the government and pharmaceutical companies have primarily discussed reimbursement by first applying it to second-line and subsequent treatments, then expanding coverage to first-line treatment. However, with recent cases demonstrating both clinical efficacy and OS benefits from first-line treatment, the prevailing opinion is that it is no longer easy to maintain this approach. For instance, Rybrevant (amivantamab, Johnson & Johnson) is an example. Recently, results from the MARIPOSA Phase 3 trial were announced, showing that the combination therapy with Rybrevant and Lazertinib (Yuhan Corp) as a first-line treatment for non-small cell lung cancer could extend OS by over 50 months. Based on these results, Johnson & Johnson applied for reimbursement for the Rybrevant-Lazertinib combination therapy as a first-line option to HIRA in the first half of this year. Therefore, the pharmaceutical industry is suggesting that the medical community and the government must find a common ground through discussions on a paradigm shift in reimbursement, starting with anti-cancer drugs. It suggests revising the reimbursement system to encourage more proactive treatment from the initial stages, shifting the focus from life extension to quality of life. An anonymous pharmaceutical industry official said, "The current reimbursement system for anticancer treatment is in a transitional period. With an increasing number of options proving treatment efficacy from the initial stages, the time to discuss reimbursement primarily for fourth or fifth-line treatments seems to have passed," and added, "It seems we are transitioning to an era where priority is given to actively treating patients early on to improve their quality of life ultimately, rather than focusing on treatments that merely extend life slightly." He added, "Currently, we are at a stage of confusion regarding the reimbursement priority between initial treatment and subsequent retrospective treatments. It's a question of whether to focus on life extension, or to recognize efficacy based on progression-free survival (PFS) in initial treatment even if OS is not extended." He stressed, "While the financial costs will undoubtedly be greater, it's now time for a specific policy direction."
- Company
- Polivy’s reimbursement for DLBLC will soon be reviewed
- by Eo, Yun-Ho Jul 15, 2025 06:08am
- Polivy, the first first-line treatment introduced for diffuse large B-cell lymphoma (DLBCL) in 20 years, is entering the first stage of its renewed bid for reimbursement in Korea. According to industry sources, Polivy (polatuzumab vedotin), a treatment developed by Roche Korea for relapsed or refractory diffuse large B-cell lymphoma (DLBCL), is expected to be reviewed by the Health Insurance Review and Assessment Service's (HIRA) Cancer Review Committee. After the company resubmitted the reimbursement application in May, the drug’s review schedule is progressing relatively quickly. Polivy originally sought reimbursement listing in 2021 for its first indication, as third-line treatment in combination with BR therapy (bendamustine and rituximab), but failed to pass the Health Insurance Review and Assessment Service's Cancer Review Committee review. Then in the first half of 2023, the company applied for reimbursement for its use as first-line treatment in combination with the so-called R-CHP therapy, which includes rituximab+cyclophosphamide, doxorubicin, and prednisone, but was again rejected by the Cancer Review Committee in February last year. Therefore, it remains to be seen whether Polivy, which has failed reimbursement listing twice in Korea, will be successful in gaining reimbursement this time. There is some optimism. The company added results from a 60.9-month follow-up analysis of the POLARIX study, which evaluated the efficacy of the Pola-R-CHP combination therapy in first-line treatment for DLBCL. The study, which was presented at the American Society of Hematology (ASH) 2024 Annual Meeting, was the first clinical trial in 20 years to expand the first-line standard of care for DLBCL. Key results showed that patients treated with the Polivy combination therapy demonstrated a significant improvement in overall survival (OS) compared to the control group treated with the standard R-CHOP. The lymphoma-related mortality rate was 9.0% in the Polivy combination therapy group and 11.4% in the R-CHOP control group. At approximately 5 years after treatment initiation, the risk of death in the Polivy combination therapy group was reduced by 15%, an improvement over the previous 3-year follow-up results (6% reduction in risk). Also, the Polivy combination therapy group (38.7%) required subsequent treatment (radiation therapy, systemic chemotherapy, CAR-T cell therapy, etc.) at a rate approximately 25% lower than the R-CHOP control group (61.7%). Diffuse large B-cell lymphoma is an aggressive form of blood cancer and the most common type of non-Hodgkin lymphoma. In South Korea, it is estimated that approximately 5,000 new patients are diagnosed with diffuse large B-cell lymphoma each year. It accounts for the highest proportion of non-Hodgkin lymphoma and is an aggressive type of lymphoma that requires immediate treatment due to its rapid progression. While over half of patients achieve remission with good treatment response rates, 30–40% of patients do not respond to standard therapy (R-CHOP) or experience recurrence after initial treatment. Despite the fact that most patients experience relapse within 2 years and have a survival period of only 6 months upon relapse, relapsed or refractory diffuse large B-cell lymphoma remains an area with limited effective treatment options.
- Policy
- Gov't pursues 'project to produce unstable supply drugs'
- by Lee, Jeong-Hwan Jul 15, 2025 06:08am
- The Ministry of Health and Welfare (MOHW) plans to continue its project of providing government funding for the production of drugs with unstable supply. The MOHW plans to proceed with the currently budgeted project to support one item, while also working to secure additional budget to support more items. A MOHW official recently met with the Korea Special Press Association to explain the direction of the project to support the production of drugs with unstable supply. The MOHW has selected 'Questran Powder for Suspension Boryung,' a bile acid sequestrant class hyperlipidemia treatment from Boryung, as an unstable supply drug to be stabilized and has decided to provide KRW 900 million in funding. This drug is the only hyperlipidemia treatment in South Korea that pregnant women and children can safely use, but its supply was halted in 2023 due to declining profitability. In response, Boryung will resume production with KRW 900 million in government support and KRW 900 million in-house investment, totaling KRW 1.8 billion. The National Assembly is also calling for budget increases in the project to support the production of drugs with unstable supply. Last year, the National Assembly's Health and Welfare Committee advocated for an additional budget increase of KRW 900 million to expand the supported items from one to two, as part of a national health security strategy to support domestic drug production and supply chains. However, the budget increase review did not happen, and the proposal was rejected. The MOHW plans to use 'Questran Powder for Suspension Boryung' as a starting point to secure the basis for the unstable drug supply support project and will maintain the project while increasing the number of supported items in the future. For 'Questran Powder for Suspension Boryung,' the company must meet a mandatory condition that it must complete the production of the requested quantity within three months if a production request from the MOHW follows within five years after the government budget support project ends. A MOHW official stated, "Only two pharmaceutical companies applied for this year's support project. It seems the promotion was insufficient." They added, "Although this is the first year of implementation, it will not be a one-off project." The official explained, "While the government provides KRW 900 million in funding and the private pharmaceutical company matches the same amount, it's not easy to fully equip production facilities with this budget. I do feel it's not a large sum," and added, "Nevertheless, I believe starting the drug supply shortage project with this item has significance." The MOHW also added, "The evaluation·verification of budget project investment returns will be based on whether the government's required production volume is met, which will be considered as having contributed to alleviating supply shortage to some extent." They concluded, "Research on resolving unstable supply chains has also been allocated a budget of KRW 50 million, and this will also begin in the second half of the year."
- Opinion
- [Reporter’s View]Biotech’s responsible approach to failure
- by Cha, Jihyun Jul 15, 2025 06:06am
- Cases of terminated licensing deals and halted clinical trials are on the rise in Korea's biotech sector. Recently, IntoCell announced that the licensing agreement for its ADC (antibody-drug conjugate) platform with ABL Bio had been canceled due to patent overlap issues. Prior to that, Orum Therapeutics voluntarily decided to halt clinical development of its lead pipeline after the occurrence of serious adverse events (SAEs). This series of announcements quickly translated into investor losses. IntoCell’s stock plummeted immediately after the termination of its licensing deal was made public. The following day, the company’s share price closed at a price 25.9% lower compared to the previous trading session. On the day of the disclosure, IntoCell hit the lower limit in after-hours trading (3:40–8:00 p.m.), falling to KRW 28,900. When Orum Therapeutics announced the suspension of its clinical trial, its stock price also fell to the lower price limit, showing a similar trend. Ironically, both companies had recently gone public via Korea’s special technology listing track (Tech Exception IPO). Less than two months after listing on the KOSDAQ, they released negative disclosures. This has fueled suspicions that the licensing deals may have been used to inflate valuations and justify higher offering prices during the IPO process. Critics have also raised concerns that the Korea Exchange (KRX) failed to properly vet these companies during the tech exception listing review process. However, it is necessary to take into account the fact that failure is inevitable in the biotech industry. New drug development is inherently a high-risk, high-reward industry with a high failure rate. Even global big pharma companies often succeed in developing only one out of dozens of candidate substances. Midway terminations of licensing agreements or discontinuation of clinical trials are not uncommon, both in Korea and abroad. In other words, failure itself cannot be regarded as the core issue. Due to the structural nature of the biotech industry, it is also unrealistic to expect the stock exchange to filter out all risks in advance. However, this does not mean that all failures should be excused. Companies must maintain a minimum level of responsibility for their technology and decision-making processes. This responsibility is even greater for publicly listed companies, as their actions can undermine trust in the entire domestic biotech industry. In fact, investors have already witnessed multiple instances where poor handling by individual companies has led to a collapse in overall market confidence. In the end, what truly matters is how a company responds after failure. The real test lies in how transparently it discloses the reasons behind a terminated clinical trial or a canceled deal, the technical limitations involved, and what alternative plans it puts forward. The greater risk is not the failure itself, but the inability to provide a convincing explanation for it. To earn a second chance in the market, companies must be willing to thoroughly explain the causes of failure and demonstrate a proactive attitude — even amid uncertainty. In this regard, IntoCell's response was noteworthy. For two days after the contract termination, IntuCell issued two official statements, sharing details on the structure and analysis of the patent issues that led to the termination, the results of legal reviews, the connection to its current pipeline, and plans to secure additional technology. The company also pledged to restore market communication and trust by having its CEO purchase KRW 10 billion worth of company shares and expanding investor relations (IR) activities. IntoCell's actions stand in contrast to some biotech companies that have remained silent or offered vague explanations in the face of failure. It remains to be seen how faithfully IntoCell will follow through on its commitments and whether this communication approach will help restore market trust in the future. Hopefully, this case will serve as a turning point for the Korean biotech industry—prompting a broader reflection on how companies confront and manage failure.
- Company
- Various immunotherapies attempt to treat Alzheimer’s
- by Son, Hyung Min Jul 15, 2025 06:06am
- A series of immunotherapy candidates are showing promise in the field of Alzheimer's disease treatment. With the limitations of existing antibody therapies becoming apparent, multinational pharmaceutical companies are attempting approaches targeting new immune pathways. In particular, pipelines targeting innate immune regulation, such as NK cell therapies and TREM2 antibodies, are gaining attention. According to industry sources on the 12th, US biotech company NKGen recently administered its autologous NK cell therapy candidate ‘troculeucel (SNK01)’ for the first time to patients with mild Alzheimer's disease who did not respond to existing antibody therapies. This is a case approved under the U.S. Food and Drug Administration's (FDA) Compassionate Use program, applied to patients whose cognitive decline persisted despite treatment with the amyloid-targeted therapy lecanemab. Troculeucel, NKGen’s cell therapy developed by using non-genetically modified ex vivo expanded autologous NK cells, is currently in a Phase IIa clinical trial in the U.S. targeting moderate patients. NKGen stated, “The therapy demonstrated efficacy in improving amyloid, tau, and alpha-synuclein levels in cerebrospinal fluid (CSF) by crossing the blood-brain barrier (BBB), as well as reducing neuroinflammatory markers such as GFAP.” In the previously conducted Phase I clinical trial, troculeucel demonstrated cognitive function stabilization and improvement in approximately 70% of patients, along with changes in biomarkers such as reduced pTau181 and GFAP levels in CSF. Treatment responses were dose-dependent, and no significant adverse reactions were observed. While existing antibody therapies have only slowed the progression of cognitive decline, NK cell therapy targets both inflammation control and protein aggregation resolution, opening up the possibility of a new mechanism of action for use. In particular, its use in patients unresponsive to existing drugs suggests potential as part of a combination strategy or as a new treatment option. NKGen Major global pharmaceutical companies have also begun full-scale development of antibody and small-molecule therapies targeting TREM2. Novartis recently began a multinational Phase II clinical trial of VHB937, a TREM2 antibody-based drug candidate, in multiple countries, including South Korea. This study is designed as a randomized, placebo-controlled trial to evaluate the efficacy and safety of VHB937 in patients with early-stage Alzheimer's disease over 72 weeks. VHB937 increases the expression of TREM2, a surface receptor on dendritic cells, and inhibits its shedding. According to the company, this strategy aims to enhance intracellular signaling, thereby simultaneously targeting the phagocytic function of microglia and anti-inflammatory effects. Sanofi recently acquired Vigil Neuroscience, a company developing a TREM2 small molecule agonist, and entered the field in earnest. VG-3927, which Vigil is developing, binds only to cell membrane receptors and does not bind to soluble TREM2, enabling more precise regulation of microglia function, which is a key differentiator. Sanofi plans to begin Phase II clinical trials of VG-3927 in the third quarter of this year. Targeting TREM2 is a mechanism of action that leading companies such as AbbVie and Takeda have experienced a series of failures. However, latecomers are shifting their focus to stabilization strategies and small molecule mechanisms after carefully analyzing the reasons for previous failures. New mechanisms continue to be developed in Korea as well In the field of Alzheimer's disease drug development, innovative approaches that go beyond the existing methods of removing amyloid beta and tau proteins and aim to regenerate and protect brain neurons are emerging. SNR1611 (generic name: trametinib), developed by the Korean drug development company Genuv, is one representative example. SNR1611 is a repurposed small-molecule compound derived from the already-approved anticancer drug trametinib. It works by inhibiting MEK1/2 enzymes within the MAPK/ERK signaling pathway. Through this mechanism, it suppresses the aberrant activation of MAPK signaling observed in neurodegenerative diseases such as Alzheimer’s disease, thereby promoting neural stem cell differentiation and the generation of new neurons. Recent preclinical studies have shown that SNR1611 promotes the expression of specific genes involved in brain neuron differentiation, resulting in a more than 2.7-fold increase in brain neural regeneration and approximately a twofold improvement in memory. These findings were published in “Experimental & Molecular Medicine,” a sister journal of the prestigious scientific journal Nature, thereby establishing scientific evidence. When SNR1611 was administered to an animal model with Alzheimer's disease, neural regeneration increased by approximately 176% and 295% in the dentate gyrus and subventricular zone, respectively, which are critical regions for memory formation. Additionally, neural regeneration effects were confirmed in the cerebral cortex, and memory improved by nearly twofold in the treatment group. Notably, SNR1611 has drawn attention for its ability to induce neurogenesis—a process previously considered nearly impossible in the adult brain—through pharmacological intervention. Genuv is also developing SNR1611 as a treatment for amyotrophic lateral sclerosis (ALS). In ALS animal models, administering SNR1611 reduced abnormal protein aggregation through autophagy activation and confirmed motor neuron protection effects. Currently, Phase 1/2a clinical trials for ALS are underway at five major hospitals in South Korea. AriBio is developing AR1001, a new drug candidate for Alzheimer's disease. In March, the company successfully transferred the technology to China's Q-BioTech. AR1001 targets multiple causes of Alzheimer's disease, including PDE5 and toxic proteins. This new drug candidate is an improved oral treatment based on mirodenafil (Mvix), a medication similar to Viagra for erectile dysfunction. Recent findings suggesting that PDE5 inhibitors like Viagra and Cialis may prevent Alzheimer's disease provide further evidence supporting the mechanism of AR1001. AR1001 is currently in Phase III clinical trials. AriBio has initiated global Phase III clinical trials in the United States, where the first patient dosing began in December 2022, as well as in Korea, the United Kingdom, Germany, France, Spain, Italy, Denmark, the Netherlands, the Czech Republic, and China.
- Company
- Omjjara’s reimbursement progress gains attention
- by Eo, Yun-Ho Jul 14, 2025 06:04am
- Attention is turning to whether progress will be made in the reimbursement listing process for the new myelofibrosis drug, Omjjara. Omjjara (momelotinib), a myelofibrosis treatment developed by GSK Korea, passed the Health Insurance Review and Assessment Service's Cancer Disease Review Committee in March and is currently awaiting submission to the Drug Reimbursement Evaluation Committee. It remains to be seen whether the HIRA process will be completed within the second half of this year. The indication currently under review is for the “treatment of myelofibrosis in intermediate- or high-risk adults with anemia.” Omjjara has a triple mechanism of action – it blocks 3 key signaling pathways, not only JAK1 and JAK2 that were inhibited by existing therapies, but also the ACVR1 (activin A receptor type). In the treatment of myelofibrosis, inhibition of JAK1 and JAK2 may contribute to improving systemic symptoms and reducing splenomegaly in patients, while inhibition of ACVR1 may help alleviate anemia by inducing a reduction in hepcidin expression. Anemia management is one of the unmet needs that remain in the treatment of existing myelofibrosis patients. Anemia, which increases blood transfusion dependency, is not merely an issue of dizziness as commonly perceived, but can lead to a life-threatening condition depending on its severity. Omjjara demonstrated significant improvements in key symptoms such as splenomegaly and a reduction in transfusion dependency in patients with anemia-associated myelofibrosis, regardless of prior JAK inhibitor treatment history, in the Phase III SIMPLIFY-1 study and the MOMENTUM study. In the SIMPLIFY-1 study, which evaluated the clinical efficacy and safety of Omjjara in comparison with Jakavi (ruxolitinib) in patients with myelofibrosis and had no prior JAK inhibitor treatment experience, Omjjara demonstrated non-inferiority to ruxolitinib in the primary endpoint of spleen volume response at 24 weeks of treatment. The proportion of patients in each arm who were transfusion-free was 66.5% in the Omjjara arm and 49.3% in the ruxolitinib arm, with significantly lower transfusion dependence (better transfusion independence) in the Omjjara arm. Seo-Yeon Ahn, Professor of Hematology at Chonnam National University Hwasun Hospital, said, “While JAK inhibitors previously used in the treatment of myelofibrosis demonstrated efficacy in reducing splenomegaly and alleviating systemic symptoms, they also posed unmet needs such as worsening anemia or increasing blood transfusion dependency. Omjjara has demonstrated significant clinical value in managing anemia, which is closely linked to the prognosis of patients with myelofibrosis. With its domestic launch, it is expected to contribute to improving treatment outcomes and quality of life for more patients."
- Company
- Aftermath of Januvia's price cut…MSD-CKD compensation fight
- by Son, Hyung Min Jul 14, 2025 06:04am
- Chon Kun Dang Regarding transaction for price difference compensation following the drug price reduction for the diabetes treatment Januvia, Chong Kun Dang and MSD Korea have not reached agreement during negotiation for nearly two years. While the distribution industry continues to demand compensation for losses incurred due to the drug price cut, the lack of clarity on who is responsible for the settlement is exacerbating confusion and burden in the field. According to industry sources on July 14, Chong Kun Dang recently sent an official letter to the Korea Pharmaceutical Distributors Association (KPDA), officially announcing its stance on the distribution industry's request for price difference compensation related to the drug price reduction of 'Januvia Family' products, including Januvia·Janumet. The distribution industry has been requesting price difference compensation from pharmaceutical companies for price differences on inventory since the sequential drug price reductions of Januvia (sitagliptin) on September 2, 2023, Janumet XR (sitagliptin·metformin) on the same date, and Janumet (sitagliptin·metformin) on October 1, 2023. The maximum price drops by dosage for each product indicate that Januvia 100mg was reduced from KRW 846 to KRW 592, Janumet XR 100/1000 mg from KRW 831 to KRW 572, and Janumet 50/1000 mg from KRW 520 to KRW 420. The maximum price difference per tablet amounted to KRW 259. However, as no compensation has been made to date, complains are growing within the industry. In response, Chong Kun Dang recently sent an official letter to the KPDA, stating that discussions regarding the responsible party have not yet concluded. According to the official letter, Chong Kun Dang acknowledges that the drug price reductions for Januvia and Janumet, due to patent expirations, were implemented sequentially between September and October 2023, and that distributors have been consistently requesting compensation. Chong Kun Dang explained that the reason no proper compensation has been made yet is a complex situation where Chong Kun Dang demanded price difference compensation from MSD Korea, the marketing authorization holder (MAH) at the time of the drug price reduction, but MSD Korea refused. Januvia is a DPP-4 inhibitor, containing sitagliptin, used to treat diabetes and is developed by MSD. Following Januvia's launch, MSD and Chong Kun Dang have been jointly promoting these products since 2016. However, in 2023, MSD Korea reorganized its chronic disease business unit to focus on its oncology and vaccine businesses, transferring the rights for the Januvia Family to Chong Kun Dang. Chong Kun Dang has been exclusively handling the domestic sales of Januvia as of July 15, 2023. MSD Korea's position is that since the marketing and revenue rights were already transferred at that time, they bear no responsibility for compensation related to the drug price reduction after September 2023, when the price cut occurred. An MSD Korea official stated, "We transferred all domestic rights, including marketing and manufacturing rights, for Januvia products to Chong Kun Dang in July 2023, transferring exclusive sales and marketing authority," and added, "Accordingly, Chong Kun Dang is responsible for price difference compensation due to drug price reductions that occurred after that point." The official added, "We are willing to compensate for inventory that we sold before July 2023, before Chong Kun Dang acquired the marketing rights." The official further explained, "It is true that the transfer of MAH was delayed until July 2024, but this was because time was needed for actual transfer preparations, such as changing product labels," and added, "At the time of the drug price reduction in September 2023, we were not engaged in actual revenue-generating activities, and all related rights had been transferred to Chong Kun Dang. The company was only the MAH on paper." Negotiations ongoing due to discrepancy between marketing rights and MAH...Distribution industry "we're troubled" The key issue is related to the division of roles and accountability between the MAH and the actual operating entity at the time of the drug price reduction. MSD Korea maintains that since it transferred domestic marketing and revenue rights for Januvia to Chong Kun Dang as of July 2023, Chong Kun Dang is responsible for price difference compensation arising from subsequent drug price reductions. Conversely, Chong Kun Dang stresses that MSD Korea still held the marketing authorization at the time of the drug price reduction. Januvia's marketing authorization was indeed transferred to Chong Kun Dang on July 23, 2024, after which the business transitioned to a global direct import structure. Chong Kun Dang believes that "Shifting the responsibility for compensation without the official change of the marketing authorization holder is unfair." However, given the sensitivity of the issue, they are taking a reserved stance by stating that it's difficult to issue an official statement and that discussions with MSD Korea are still ongoing. In 2016, MSD Korea and Chong Kun Dang have signed an agreement to jointly promote treatments for diabetes and dyslipidemia, including Januvia. However, as negotiations delay, the burden of price difference compensation due to the drug price reduction is ultimately being passed on to pharmaceutical distributors. The pharmaceutical distribution industry has consistently demanded compensation for the drug price difference of Januvia family products since the second half of 2023. However, settlement has been delayed due to the ambiguity of accountability between the two companies. The recent official letter sent by Chong Kun Dang to the KPDA directly mentioned this dissatisfaction from the distribution industry. The letter stated, "Discussions between the two companies are ongoing, but as the compensation discussion has not been finalized, distributors are facing difficulties," adding, "We will proactively work towards a swift resolution with responsibility." An official from a pharmaceutical distribution company pointed out, "The pharmaceutical companies that should be responsible for settling the price difference due to the government's drug price reduction are pushing responsibility onto each other," and added, "Distributors compensate pharmacies for the price difference but receive no compensation from the pharmaceutical companies, thus bearing the entire burden in the middle." The industry widely expects the transfer of Janumet XR's marketing authorization in November, raising concerns that the same problem could recur. If the discussion over responsibility drags on while the drug prices of major sitagliptin-based diabetes treatments like Januvia, Janumet, and Janumet XR are sequentially reduced. In that case, distributors will inevitably continue to bear the settlement burden. Ultimately, this discussion goes beyond individual products and extends to the industry-wide challenge of how to resolve systemic blind spots that can arise from the separate structures of marketing rights and marketing authorization.
- Policy
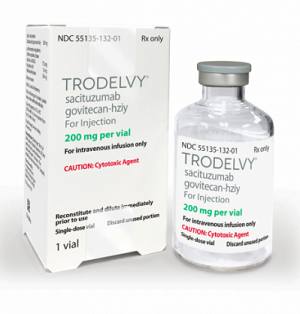
- How Trodelvy was applied flexible ICER for innovativeness
- by Lee, Tak-Sun Jul 14, 2025 06:04am
- The ADC breast cancer drug Trodelvy (sacituzumab govitecan, Gilead), which was reimbursed in June for triple-negative breast cancer, was the first case in which the ICER (incremental cost-effectiveness ratio) threshold was flexibly applied in recognition of its innovation. So how did the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service at the time evaluate the innovativeness of this drug? According to the recently released evaluation results, Trodelvy was found to satisfy all criteria for innovative treatment, including the absence of alternative treatments, clinical improvement, and the criteria for expedited review. According to industry sources on the 11th, in the recently released DREC evaluation results for Trodelvy, the committee members recognized its reimbursement as appropriate despite the high cost-effectiveness ratio, taking into account the drug’s innovativeness." In August last year, the Health Insurance Review and Assessment Service established new requirements for the innovation of drugs subject to flexible ICER threshold assessment. According to the newly established criteria, the innovation of a new drug is recognized when ▲there are no substitute products or treatments with equivalent therapeutic positions, ▲significant clinical improvement can be recognized in the final outcome indicators, such as prolonged survival, ▲the new drug is a new drug approved by the Ministry of Food and Drug Safety through an expedited review process in accordance with Article 35-4, Paragraph 2 of the Pharmaceutical Affairs Act, or a drug recognized by the committee as equivalent to such a drug. All three requirements must be met for a new drug to be recognized as innovative. Trodelvy became the first drug to be recognized as innovative under these criteria. At the time, the DREC members evaluated that Trodelvy satisfied all three requirements. First, they determined that it satisfied the first requirement as the first antibody-drug conjugate (ADC) approved for triple-negative breast cancer, with no products or treatments with equivalent therapeutic positions. In addition, they evaluated that the second requirement was also satisfied, as clinical improvement (mOS HR=0.51) was recognized in the final outcome indicators, such as prolonged survival. Finally, considering that it was approved by the FDA as a breakthrough therapy and designated as a Global Innovative product for Fast Track item by the MFDS, the committee deemed that the third requirement was also satisfied. In terms of cost-effectiveness, the committee concluded that Trodelvy is significantly more cost-effective than existing anticancer drugs and breast cancer drugs that have been reviewed in the past. However, considering that it is a drug used for diseases that threaten survival in the third or further line of administration, that it has been recognized for improving the social burden of disease and quality of life, and that it is the first antibody-drug conjugate (ADC) approved for triple-negative breast cancer, the committee accepted its reimbursement as adequate. In other words, the high price was accepted in recognition of its innovation. Another factor was that the number of patients eligible for treatment was small, so the drug’s financial impact was not significant. According to the DREC evaluation results, Trodelvy is a drug used by approximately 200 patients, and the decision was made after comprehensive consideration of factors such as the number of patients eligible for treatment and the financial impact. In the end, Trodelvy was listed for reimbursement under two types of risk-sharing agreements (RSA) with the National Health Insurance Service. Among the types of RSA contracts that were applied, one was a Refund-Type agreement, where the pharmaceutical company reimburses a certain percentage of the claimed amount to the NHIS, and the other was the Utilization Cap/Fixed Cost per Patient Refund Type agreement, where the usage limit per patient is set in advance, and a certain percentage of the excess amount is reimbursed to the NHIS if the limit is exceeded. The listed price (maximum amount) is KRW 1,052,300 per vial, but due to the dual pricing system applied under the reimbursement-type contract, the actual price is higher. The estimated number of patients receiving Trodelvy annually is approximately 282, with an estimated fiscal expenditure of approximately KRW 12.5 billion. However, the actual fiscal expenditure is expected to be lower if the risk-sharing agreement is applied. At a 5% coinsurance rate, patients will only need to pay approximately KRW 2.21 million per year for its administration.
- Company
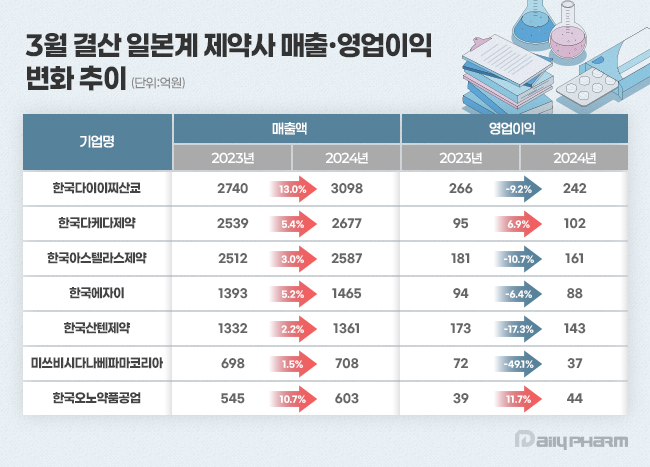
- Daiichi Sankyo and Ono Pharmaceutical see surge in sales
- by Son, Hyung Min Jul 14, 2025 06:03am
- Japanese pharmaceutical companies operating in South Korea saw overall sales growth last year. All seven companies surveyed achieved year-on-year sales growth, with Daiichi Sankyo Korea and Ono Pharma Korea leading the way with double-digit growth rates. However, in terms of operating profits, the companies showed mixed emotions. According to the Financial Supervisory Service's electronic disclosure system on the 11th, the performance of 7 Japanese pharmaceutical companies with March fiscal year-ends totaled at KRW 1.2499 trillion, up 6.3% from KRW 1.1759 trillion the previous year. During the same period, operating profit decreased by 11.2% from KRW 92 billion to KRW 81.7 billion. Major Japanese pharmaceutical companies' Korean subsidiaries follow the Japanese fiscal year, recognizing the period from April last year to March this year as their 2024 annual sales. #SB Daiichi’s sales surpass KRW 300 billion for the first time... Ono’s sales double in four years Last year, Daiichi Sankyo Korea's sales increased by 13.0% year-on-year to KRW 309.8 billion. During the same period, operating profit decreased by 9.2% from KRW 26.6 billion to KRW 24.2 billion. Daiichi Sankyo Korea's sales have been steadily increasing since 2020. The company surpassed KRW 200 billion in sales for the first time in 2020 with KRW 217.9 billion, followed by KRW 245.4 billion in 2021, KRW 253.2 billion in 2022, and KRW 274 billion in 2023, continuing its upward trend. In particular, it is analyzed that the company achieved synergistic effects by collaborating with the domestic pharmaceutical company Daewoong Pharmaceutical on certain cardiovascular products such as Lixiana and Sevikar. Daiichi Sankyo Korea signed a co-marketing agreement with Daewoong Pharmaceutical for Sevikar in 2013 and Lixiana in 2015, and has maintained its partnership with the company to date. Among these, the highest-selling product is the direct-acting oral anticoagulant (DOAC) Lixiana. According to market research firm UBIST, Lixiana's prescription sales last year reached KRW 117.5 billion, an 11.6% increase from KRW 105.3 billion in 2023. The three-component hypertension combination drug Sevikar HCT also maintained its growth momentum. Last year, Sevikar HCT's prescription sales reached KRW 42.1 billion, up 4.0% from the previous year. Daiichi Sankyo Korea generated approximately KRW 140 billion in prescription sales from olmesartan-based hypertension treatments, including Sevikar HCT (KRW 42.1 billion), Olmetec (KRW 30.6 billion), and Sevikar (KRW 68.8 billion). Daiichi Sankyo Korea is working to expand its business from cardiovascular drugs to anticancer drugs. In particular, the company is focusing on new growth areas such as antibody-drug conjugates (ADCs) by concentrating its R&D capabilities. Following the approval of Enhertu, the company is preparing to launch various treatments, including five ADC strategies such as Datroway, Patritumab deruxtecan, DS-7300, and DS-6000. Last year, Takeda Pharmaceutical Korea's sales increased by 5.4% year-on-year to KRW 267.7 billion. Operating profit rose by 6.9% to KRW 10.2 billion. Anticancer drugs such as Zejula, Alunbrig, and Ninlaro contributed to the sales growth through steady growth. According to the market research firm UBIST, Alunbrig recorded prescription sales of KRW 12.1 billion last year. Since its launch in 2019, its prescription sales have continued to increase. This treatment is gaining market share for its indication in ALK-positive non-small cell lung cancer. Alunbrig is in fierce competition with Pfizer's Lorviqua and Roche's Alecensa. However, However, Alunbrig offers the advantage of once-daily, single-tablet dosing, and can be taken regardless of food intake. This simplicity reduces the number of factors patients need to consider when taking the medication. The ovarian cancer treatment Zejula also recorded KRW 14.3 billion in sales last year, a 51.6% increase in prescriptions from the previous year. In October last year, Zejula was added to the health insurance reimbursement list for the treatment of HRd-positive ovarian cancer. Until then, Zejula had been reimbursed only as maintenance therapy for BRCA-mutated ovarian cancer patients who responded to platinum-based therapy as a first-line treatment for ovarian cancer. The growth of Ono Pharma Korea was also noteworthy. Ono’s sales last year were KRW 60.3 billion, up 10.7% year-on-year. Operating profit in the same period increased 11.7% from KRW 3.9 billion to KRW 4.4 billion. The sales and operating profit recorded by Ono last year are the highest figures since the company began submitting audit reports in 2017. Ono recorded sales of over KRW 40 billion in 2021 and surpassed KRW 50 billion for the first time the following year. Comparing the KRW 60.3 billion recorded by Ono last year with the KRW 31 billion in sales in 2020, sales increased by 94.5% over four years. The immune-oncology drug Opdivo drove Ono’s sales growth. Opdivo is an anti-PD-1 class immuno-oncology drug jointly developed by Ono and BMS, and was approved in Korea in 2015. Ono holds development and sales rights in Asia, including South Korea, Japan, and Taiwan. In particular, the combination of Opdivo and BMS's CTLA-4 targeted immunotherapy Yervoy has demonstrated efficacy in various solid tumors such as melanoma, renal cell carcinoma, and hepatocellular carcinoma, contributing to the increase in market share. According to market research firm IQVIA, Opdivo surpassed KRW 100 billion in domestic sales in 2022. All showed external growth, but with mixed operating profits Astellas Pharma Korea, Eisai Korea, and Santen Pharmaceutical Korea all saw slight increases in sales, but their operating profits all declined. Astellas Pharma Korea reported sales of KRW 258.7 billion last year, a 3.0% increase from the previous year. Harnal, Betmiga, Prograf, and Xtandi performed well, driving sales growth. Harunal, a treatment for benign prostatic hyperplasia, recorded prescription sales of KRW 66.2 billion last year, a 0.8% increase from the previous year. Betmiga, a treatment for overactive bladder, and Prograf, an immunosuppressant, have consistently recorded prescription sales of over KRW 30 billion even after their patents expired. Last year, prescription sales for Betmiga and Prograf were KRW 33.4 billion and KRW 33.6 billion, respectively, up 2.6% and 3.9% year-over-year. Operating profit decreased by 10.7% year-on-year to KRW 16.1 billion. A slight increase in the cost of sales contributed to the decrease in gross profit. Astellas Pharma Korea is pinning its hopes on its next-generation anticancer drugs. The company's Padcev, its ADC anticancer drug, and Vyloy, its new gastric cancer targeted therapy targeting Claudin 18.2, have entered the domestic market. Both drugs have shown remarkable results in clinical trials. Currently, Astellas Pharma Korea has completed its application for insurance reimbursement for both drugs. Eisai Korea's sales rose 5.2% from KRW 139.3 billion in 2023 to KRW 146.5 billion last year. Operating profit for the same period decreased 6.4% to KRW 8.8 billion. Sales of existing products such as the anticancer drug Lenvima, the gastroesophageal reflux disease treatment Pariet, and the Alzheimer's disease treatment Aricept remain steady. The three products—Aricept (KRW 95.9 billion), Pariet (KRW 17.9 billion), and Lenvima (KRW 12.9 billion)—combined for over KRW 100 billion in sales last year. Operating profit decreased slightly due to factors such as increased cost of sales. EisaiEisai is expecting strong performance from its new Alzheimer's disease drug, lecanemab. Lecanemab is an Alzheimer's disease treatment developed by Eisai and Biogen that selectively binds to amyloid beta, a substance believed to cause the disease, and has been proven to slow the progression of the disease and delay cognitive decline. It is reported that lecanemab is currently being prescribed at various general hospitals and semi-general hospitals in Korea. Santen Korea reported sales of KRW 136.1 billion last year, a 2.2% increase from the previous year. However, operating profit decreased by 17.3% to KRW 14.3 billion. Santen specializes in ophthalmic products and holds various formulations, including diquafosol. However, in South Korea, hyaluronic acid eye drops are primarily used, and the company has not significantly increased its prescription volume in Korea. As of last year, the product with the highest sales among Santen Pharmaceutical's portfolio was Cosopt S eye drops, used for glaucoma. Cosopt S’s prescription volume last year was KRW 33.9 billion, an increase of 2.6% compared to the previous year. Mitsubishi Tanabe Pharma Korea's sales last year were KRW 70.8 billion, up 1.5% from 2023, but operating profit fell 49.1% in the same period. The decrease in operating profit was due to an increase in selling, general, and administrative expenses, such as reimbursement and retirement benefits, as well as an increase in cost of sales.