- LOGIN
- MemberShip
- 2025-12-17 20:36:33
- Opinion
- [Reporter’s View] What about the ‘everyday’ drugs?
- by Kim, Jin-Gu Dec 15, 2025 11:03am
- The government’s newly announced drug pricing reform plan clearly reflects its intention to restructure Korea’s pharmaceutical and biotech industry: reward innovative new drugs, streamline generics, and reduce the public’s drug expenditure burden.However, one critical element is conspicuously absent—supply stability. The ‘supply’ issue, which directly impacts the site and the patients, has not been sufficiently addressed. While an item titled ‘Establishing a Stable Supply System for Essential Medicines’ exists midway through the reform plan, the overall weight given to this area appears insufficient.In recent years, the medical field has suffered from repeated drug supply disruptions. Antibiotic shortages paralyzed pediatric care, while fever and pain reliever shortages made pharmacies reminiscent of rationing. Medical institutions have repeatedly sweated bullets securing substitutes as essential medicines like contrast agents, local anesthetics, and basic IV fluids that are indispensable for treatment, have repeatedly run out. Supply instability has spread beyond specific essential medicines to encompass ‘everyday drugs’ used in routine care, exposing structural weaknesses in Korea’s pharmaceutical supply chain.Of course, supply stabilization measures are included in this reform plan. The threshold for designating exit-prevention drugs was raised by 10%, and the cost recovery standard for low-priced drugs was expanded from an annual claim amount of KRW 100 million to KRW 500 million. A new policy surcharge of up to 7% will be introduced, and the calculation methods for manufacturing and labor costs will be adjusted to reflect reality. For essential medicines, the surcharge pricing period will be stably guaranteed, and the scope of surcharge eligibility will be expanded. A ‘reshoring surcharge’ will also be considered when switching imported items to domestic production. Furthermore, the government will strengthen the public-private joint response system and push to establish a system that automatically guides users to substitute items during the prescription and dispensing stages.However, the pharmaceutical industry's response remains lukewarm. This is because the revised plan still fails to provide pharmaceutical companies with a ‘clear reason’ compelling them to produce low-cost essential medicines. Raw material costs and fixed expenses rise every year, yet numerous items have seen drug prices stagnate for years. Critics argue that limited price incentives confined to specific items cannot resolve structural issues. While plans exist to encourage compliance with supply commitments, encouragement alone cannot sustain manufacturing lines.For these reasons, the reform plan reads like a structure sacrificing fundamentals for innovation. The medicines citizens take daily, the drugs used in emergency rooms, and the medications needed on hospital wards are not mere pharmaceuticals but the very ‘medical infrastructure’ itself. No matter how many innovative new drugs emerge, if the supply of ‘everyday medicines’ – the foundation of the healthcare system – remains unstable, the impact of policy reforms will inevitably fall short.The direction hereon is clear. We must elevate the stability of pharmaceutical supply from a ‘side clause’ in drug pricing system reforms to a ‘core pillar’. Practical and sustained incentives are needed to encourage pharmaceutical companies to compete for the production of essential medicines. Beyond minor adjustments that merely cover costs, we must design realistic and sustainable profit structures that ensure supply continuity. System-level frameworks to support this also require an overhaul.Developing global innovative new drugs is important. However, safeguarding the supply chain to prevent disruptions in medicines that patients urgently need must take precedence over any other policy. What the pharmaceutical industry, the medical community, and patients ultimately desire is a policy that solidly establishes these fundamentals. This is precisely the part the current reform plan has overlooked.
- Opinion
- [Reporter's View] Besides speeding reform of pricing system
- by Jung, Heung-Jun Dec 15, 2025 11:03am
- If the government's goal for reforming the drug pricing system is to improve structure of the pharmaceutical and biotech industry, then an appropriate processing speed is necessary.To encourage pharmaceutical companies to expand R&D investment and align their interests, the government must provide sufficient time for companies to devise new plans.The government will gather industry-wise opinions and finalize the pricing system reform by January. Then, it plans to implement the policy in July, half a year later. The schedule is too fast-paced, as concerns arise about potential exhaustion rather than improvements to the industry structure. The Ministry of Health and Welfare (MOHW) has repeatedly stated that the system reform is not intended to achieve pharmaceutical cost savings. The MOHW asks that the reform, which has been designed to encourage innovations, should be viewed as an attempt to establish South Korea as a strong nation for new drugs.The question is whether companies devising plans in a hurry following the Health Insurance Policy Review Committee's decision in February next year can bring innovation to the industry? Even if the rate of R&D investment can be increased, the policy is highly likely to be a half-baked deal to maximize drug pricing.Furthermore, the greed to reach the goal in a short period may be the underlying reason that pharmaceutical companies with high potential for change give up.The government has suggested direction of the drug pricing system. Now, it should give enough time for the industry to reorganize portfolio and devise 5- to 10-year plans. Six months is too short for the industry to finalize revision measures.If the government were to ignore the industry's reality and focus solely on the roadmap for system implementation, there may be unintended consequences beyond the expected goals.Besides the industry, the government must consider well-designed implementation measures to achieve both goals, including innovation and stable supply.There are concerns about anticipated side effects of changes to the post-marketing management system, including the side effects of simply offering drug pricing priority based on R&D rate or actual transaction price survey.Once the post-marketing monitoring system is reduced to twice a year, remaining tasks to specify include how to permit retroactive application or how to promote listing within 100 days for rare disease pharmaceuticals.It is difficult to build a healthy ecosystem. It carries the danger of collapsing the existing ecosystem while failing to establish the intended ecosystem. Once collapsed, an ecosystem requires substantial financial resources.Setting the right direction and improving the system requires the government's firm stance. However, as this is a critical decision impacting the industry and future business, the government should take the time to carefully review the policy, while the industry works to monitor and develop effective plans.
- InterView
- ‘Imfinzi demonstrates rationale for perioperative immunotherapy in gastric cancer’
- by Son, Hyung Min Dec 15, 2025 11:02am
- The role of adjuvant systemic therapy is emerging as an important consideration even in patients with resectable gastric and gastroesophageal junction (GEJ) adenocarcinoma.Despite outstanding surgical outcomes in East Asia, a significant proportion of patients with stage II-III locally advanced disease still experience recurrence due to micrometastases. In this context, a perioperative treatment strategy, which involves systemic therapy before and after surgery, has emerged as a promising approach to enhance long-term outcomes.At the recent ESMO Asia Congress 2025 held in Singapore, results from an Asian subgroup analysis of the Phase III MATTERHORN study were presented, reinforcing the clinical value of Imfinzi (durvalumab) in combination with standard FLOT chemotherapy (5-FU, leucovorin, oxaliplatin, and docetaxel).In the study, Imfinzi plus FLOT demonstrated clinically meaningful improvements in event-free survival (EFS), 3-year overall survival (OS), and pathological complete response (pCR). These findings signal a clear shift toward earlier use of immunotherapy in resectable gastric cancer.Surgery remains the cornerstone of the cure in gastric cancer. However, there is growing global consensus, including in Asia, that surgery alone is insufficient for many patients. The MATTERHORN study demonstrated that administering a combination of chemotherapy and immunotherapy prior to surgery, followed by curative resection and additional therapy afterward, can significantly improve long-term outcomes.Based on these results, the U.S. Food and Drug Administration (FDA) approved Imfinzi for the treatment of adult patients with resectable, early-stage, and locally advanced (stages II, III, and IVA) gastric and gastroesophageal junction (GEJ) cancers last month. The approved regimen includes neoadjuvant Imfinzi in combination with chemotherapy before surgery, followed by adjuvant Imfinzi in combination with chemotherapy, then Imfinzi monotherapy.At ESMO Asia 2025, Daily Pharm spoke with Dr. Yelena Y. Janjigian, first author of the MATTERHORN study (Chief of the Gastrointestinal Oncology Service at Memorial Sloan Kettering Cancer Center), as well as Professor Sun Young Rha of Yonsei Cancer Center (President of the Korean Cancer Association) to discuss the clinical implications of this shift and the challenges associated with its implementation in Korea.Q. As the first author of MATTERHORN, what prompted the development of this perioperative immunotherapy strategy for patients with resectable gastric and GEJ adenocarcinoma, and what is the clinical significance of the results?Professor Yelena JanjigianDr. Yelena Janjigian: MATTERHORN holds significance as the first Phase III study in the resectable, early-stage setting to demonstrate improvements across pCR, EFS, and OS with chemo-immunotherapy.At ESMO Asia, we presented subgroup results from Japan, Korea, and Taiwan, which were consistent with the global findings. These data support the global relevance of the perioperative approach.With recent FDA approval for resectable gastric and GEJ adenocarcinoma, aligning treatment approaches across regions will help us move the field forward, especially given the global incidence of more than 1.2 million new cases each year.Q. The Asian subgroup showed efficacy and safety trends consistent with global results.Professor Yelena Janjigian: Three key points are important. First is the feasibility across regions. Perioperative Imfinzi was deliverable in Asian centers, and physicians were able to administer both FLOT and durvalumab safely.It also did not compromise surgical care. Rates of complete (R0) resection and the ability to proceed to surgery were maintained.Furthermore, the results showed improvement across all major efficacy endpoints—pCR, EFS, and OS. The ability to improve all three endpoints in one study is unprecedented in this setting and underscores the strength and value of the data.Q. What considerations should clinicians keep in mind when applying the regimen in earlier-stage patients?Professor Yelena Janjigian: The study enrolled a broad age range—from 18 to 84 years—and older patients benefited similarly to younger patients.That said, some patients, particularly in Asian populations, may present with nutritional compromise or lower baseline white blood cell counts. Asian investigators are familiar with these characteristics. Strategies such as early G-CSF administration and careful initial dose tailoring of FLOT can help maintain safety while preserving efficacy.Most importantly, close clinical monitoring, hydration, and supportive care are essential components of successful perioperative treatment.Q. Some Korean clinicians believe the regimen may be more suitable for stage III or IV than stage II. What is your view on this?Professor Yelena Janjigian: Clinical staging in gastric cancer is often imprecise. A patient staged clinically as T2 may be found at surgery to have T3N1 disease. Because many patients have difficulty tolerating intensive postoperative therapy after major gastrectomy, preoperative treatment becomes particularly valuable.We believe the greatest benefit of chemo-immunotherapy occurs when the tumor remains in place, allowing optimal priming and expansion of anti-tumor T-cells. This is supported by the stronger efficacy observed in the neoadjuvant component compared with adjuvant-only settings.In my clinical practice, even patients with clinically estimated T2 tumors—especially those with diffuse or signet-ring histology—are discussed in a multidisciplinary context for consideration of perioperative systemic therapy.Additionally, the shift in tumor epidemiology in Asia—with increasing proximal and GEJ tumors—further supports the need for downstaging approaches, as these tumors historically have poorer surgical outcomes.Q. How should we interpret the potential for non-operative management in early gastric cancer?Professor Yelena Janjigian: This area is highly exploratory and remains limited to very select patients within clinical trials or specialized centers.In certain patients who achieve a complete clinical and pathologic response and who can reliably adhere to intensive surveillance, non-operative strategies have been examined. However, this is not standard practice and remains controversial, particularly among surgeons.Nevertheless, these discussions reflect a broader evolution toward balancing cure with long-term quality of life. Gastrectomy, even when minimally invasive, alters eating patterns, sleep patterns, and body image. For selected patients, preserving the stomach—when supported by rigorous evidence and strict monitoring—could represent a meaningful advance, but this requires further research before it can be broadly implemented.Q. Gastric cancer has historically been viewed as a disease with favorable surgical outcomes, leading to a lesser emphasis on chemotherapy. Yet MATTERHORN highlights the importance of perioperative treatment. Why is this approach necessary?라선영 연세암병원 교수Professor Sun Young Rha: MATTERHORN enrolled patients with stage II and III gastric cancer eligible for curative surgery. While stage I “early gastric cancer” is often cured through endoscopic treatment or gastrectomy, stage II–III cancers are locally advanced and carry significantly higher recurrence risk.In East Asia, surgery followed by adjuvant chemotherapy has historically yielded strong outcomes, with five-year survival rates around 75–80%. Still, approximately 30–40% of stage III patients experience disease relapse, demonstrating a significant unmet need.Perioperative therapy aims to eradicate micrometastases after surgery and sustain systemic control afterward. In Western countries, perioperative FLOT has become the standard to achieve a better tumor resection, considering factors such as the increased obese population and different tumor extent at GEJ.Q. What are the key findings of MATTERHORN, and which patients are most likely to benefit from D-FLOT?Professor Sun Young Rha: The MATTERHORN study is significant in that it introduces a new treatment option for patients with locally advanced, resectable gastric cancer. Notably, a distinct survival benefit was observed in high-risk stage III patients with a high tumor burden. It is also noteworthy that the overall survival curve suggests the possibility that more than half of patients may remain alive at five years.However, it should be noted that MATTERHORN did not compare the experimental arm with the post-operative adjuvant chemotherapy that has been long used in Korea or Japan, but rather with FLOT, which is considered the global standard of care. While FLOT is highly effective, Asian patients may encounter challenges in completing treatment due to its relatively high intensity.Therefore, especially in Asia where surgical outcomes are generally more favorable, it may be more appropriate to selectively apply this regimen to patients with high-stage disease.Q. If the D-FLOT regimen becomes available in Korea, which patients would be the most appropriate candidates?Professor Sun Young Rha: If ongoing follow-up continues to support the favorable outcomes observed in stage III patients—particularly in terms of EFS and 3-year OS—this group will likely be prioritized in Korea.For stage II patients, surgery followed by adjuvant chemotherapy already yields a 5-year OS rate up to 80%, and the potential incremental benefit of perioperative D-FLOT must be balanced against its higher treatment burden and toxicity profile.In MATTERHORN, approximately 40–50% of patients completed the full perioperative regimen, reflecting the challenges associated with the adjuvant FLOT component. This underscores the importance of careful patient selection. Stage III patients, who have higher rates of micrometastatic disease and a 30–40% recurrence risk within two years, are expected to derive the greatest benefit.Clinical features such as extensive nodal involvement, T4 tumors, or biologically aggressive histology should guide decision-making. Biomarker-informed approaches will increasingly help tailor treatment intensity and minimize unnecessary toxicity.Q4. What distinguishes Asian patients from Western patients in MATTERHORN?Professor Sun Young Rha: A notable feature of Asian patients enrolled in the MATTERHORN study is that they generally had good performance status and a relatively lower tumor burden. In countries such as Korea and Japan, where early detection rates are high and surgical outcomes are excellent, patients enrolled in clinical trials can potentially have better overall performance status compared to their Western counterparts.While the proportion of stage IV patients in the study appears somewhat high, it should be interpreted as including patients with high-risk, advanced gastric cancer characterized by deep tumor invasion or extensive lymph node involvement, rather than those with unresectable or incurable M1 metastatic disease.In the MATTERHORN study, Asian patients represented approximately 20% of the total population, indicating that additional real-world experience will be needed to further strengthen the evidence base. It will be important to continue accumulating data from Asian patients to more clearly define the clinical significance in future practice.Q. What challenges must Korea overcome to adopt perioperative durvalumab?Professor Sun Young Rha: Above all, aligning treatment approaches between surgeons and medical oncologists is essential. Especially in Korea and Japan, where surgical outcomes are outstanding, and the paradigm remains surgery-centric. Consensus-driven discussions with surgeons are needed to establish the role of perioperative immunotherapy in high-risk advanced gastric cancer. To ensure patients do not lose access to optimal treatment options, multidisciplinary collaboration is vital for designing comprehensive treatment plans.From a medical oncology standpoint, individualized dose adjustment and proactive management of adverse events are key, particularly given the complexity and toxicity of the FLOT regimen. Active supportive care (such as antiemetics, G-CSF support, and dose modification) is necessary to help Asian patients complete therapy.Finally, reimbursement is a major hurdle. Without sustainable reimbursement pathways, early real-world adoption will be limited. Generating clinical experience and real-world data, especially in high-risk stage III patients who are most likely to benefit, is important to help establish the evidence base needed for broader access.
- Policy
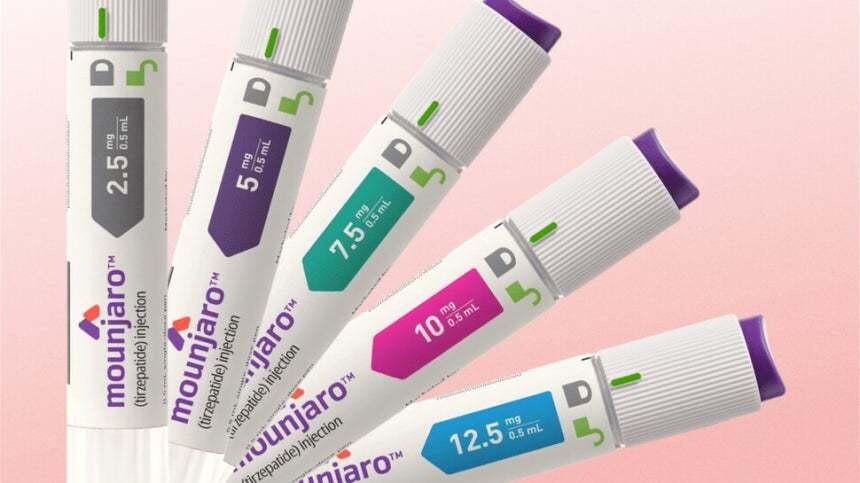
- Vial formulation of Mounjaro approved only for diabetes in KOR
- by Lee, Tak-Sun Dec 15, 2025 09:16am
- Lilly’s ‘Mounjaro Prefilled Pen Inj’Lilly has obtained Korean regulatory approval for a vial formulation of Mounjaro in Korea. Mounjaro is a GLP-1 and GIP dual agonist, and its prefilled pen formulation was launched in August as an obesity treatment.The Ministry of Food and Drug Safety approved six dosage strengths of Mounjaro Vial Inj (tirzepatide) on the 12th. Like the previously approved pre-filled pen, it comes in 2.5mg/0.5ml, 5mg/0.5ml, 7.5mg/0.5ml, 10mg/0.5ml, 12.5mg/0.5ml, and 15mg/0.5ml doses.However, its indication for chronic weight management in adults was excluded. The newly approved Mounjaro vial formulation is indicated solely as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes, either as monotherapy or in combination therapy.Earlier this month, Mounjaro Prefilled Pen received a positive reimbursement assessment as a diabetes treatment from the Drug Reimbursement Evaluation Committee (DREC) under Korea’s Health Insurance Review & Assessment Service (HIRA). Products that pass the DREC review proceed to price negotiations with the National Health Insurance Service (NHIS), followed by final listing approval by the Health Insurance Policy Deliberation Committee.With reimbursement listing now highly likely for the prefilled pen formulation, Lilly is expected to pursue reimbursement for the vial injection formulation as well.Lilly secured three formulations: Mounjaro Prefilled Pen in June 2023, Mounjaro QuickPen in September this year, and now Mounjaro vial formulation.The currently marketed formulation is the prefilled pen formulation, supplied without reimbursement as an obesity treatment. Mounjaro generated sales of KRW 28.4 billion (IQVIA) within two months of launch, fueling strong market momentum alongside Wegovy.Tirzepatide, the active ingredient in Mounjaro, selectively binds to and activates both GIP and GLP-1 receptors, which are targets of endogenous incretin hormones. Through this mechanism of action, it enhances first- and second-phase insulin secretion in a glucose-dependent manner and suppresses glucagon secretion. GLP-1 is a key physiological regulator of appetite and calorie intake.
- Company
- JP’s Mitsubishi Tanabe Pharma name changes to 'Tanabe Pharma'
- by Eo, Yun-Ho Dec 15, 2025 09:16am
- Mitsubishi Tanabe Pharma has changed its company name to Tanabe Pharma.Accordingly, the Korean subsidiary was officially re-branded as Tanabe Pharma Korea (TPKR) as of the 1st of this month. This decision was made at the parent company's recent extraordinary general meeting of shareholders.Mitsubishi Chemical Group sold Mitsubishi Tanabe Pharma to the U.S. private equity firm Bain Capital earlier in February. Mitsubishi Chemical Group plans to withdraw from the pharmaceutical business, which requires massive R&D spending, and focus its management resources on its core businesses.Tanabe Pharma Korea plans to continue its activities to ensure a smooth supply of medicines to the Korean market, irrespective of the name change.Tanabe Pharma Korea recently secured market approval for Radicut Suspension, the oral formulation of the ALS (Amyotrophic Lateral Sclerosis) treatment Radicut. It is also proceeding with the reimbursement listing process for Uplizna (inebilizumab), a treatment for anti-aquaporin-4 (AQP4) antibody-positive adult patients with Neuromyelitis Optica Spectrum Disorder (NMOSD).Meanwhile, Tanabe Pharma was initially established in 1678. It reached its current form after merging with Mitsubishi Pharma, a subsidiary of Japan's largest chemical group, Mitsubishi Chemical Group, in 2007.
- Company
- "Securing global CDMO foundation in KOR"
- by Kim, Jin-Gu Dec 15, 2025 09:16am
- (from left) Yong Ki Park (Project Manager, Samsung Biologics), Yoon Hee Choi (Senior Research Fellow, KIET), Sun Hee Lee (Professor, Ewha Womans University), Tae-kyu Lee (CEO, Scale Up Partners), Tong Kook Lee (Attorney, Dongin Law Group Lawyer), Kang Seop Im (Head, Ministry of Health and Welfare's Division of Health Industry Promotion)The Korea Pharmaceutical and Bio-Pharma Healthcare Alliance, an assembly of organizations representing South Korea's domestic pharmaceutical, bio, and medical device sectors, held its second official Forum. At the event, the industry voiced for regulatory rationalization and expanded nation-level investment in manufacturing infrastructure to elevate domestic capabilities to a global standard.The Korea Pharmaceutical and Bio-Pharma Healthcare Alliance hosted its 2nd Forum on December 11 at the National Assembly Hall, focusing on the topic of 'manufacturing innovation strategy for the healthcare industry to enhance global competitiveness'.The Korea Pharmaceutical and Bio-Pharma Healthcare Alliance is a healthcare consultative body formed by key associations representing the South Korean healthcare industry, including pharmaceuticals, medical devices, regenerative medicine, and digital health. Nine organizations have joined the alliance, including the Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA), Korea Biomedicine Industry Association, Korea Pharmaceutical Traders Association(KPTA), Korea Drug Research Association (KDRA), Council for Advanced Regenerative Medicine (CARM), Korea Digital Health Industry Association(KoDHIA), Korea Medical Devices Industry Association (KMDIA), and Korea Biotech Industry Organization.The forum was attended by heads of alliance member institutions, including Yunhong Noh (President, KPBMA), Jung Jin Kim (Chairman, KDRA), Jeong Seok Lee (Chairman, KBMIA), Young Min Kim (Chairman, KMDIA), Byoung-jun Bae (Chairman, CARM), Ki-yoon Yoo (Executive Director, KoDHIA), and Dong Hee Lee (Vice President, KPTA).Panelists included Sun Hee Lee (Professor, Ewha Womans University), Yong Ki Park (Project Manager, Samsung Biologics), Tong Kook Lee (Attorney, Dongin Law Group Lawyer), Tae-kyu Lee (CEO, Scale Up Partners), Kang Seop Im (Head, Ministry of Health and Welfare's Division of Health Industry Promotion), and Yoon Hee Choi (Senior Research Fellow, KIET), discussing strategies for domestic healthcare manufacturing innovation and strengthening national Contract Development and Manufacturing Organization (CDMO) competitiveness. The Korea Pharmaceutical and Bio-Pharma Healthcare Alliance hosted its 2nd Forum on December 11 at the National Assembly Hall, focusing on the topic of 'manufacturing innovation strategy for the healthcare industry to enhance global competitiveness'.Jung Woon Chun, a researcher at the KPBMA, assessed that domestic pharmaceutical and bio manufacturing and quality competitiveness remain at an early stage, with distinct disparities by company size. Researcher Chun noted, "According to a survey conducted by the association involving 45 companies and 61 plants, the automation level of domestic companies remains at the MES/ERP implementation stage (Level 2), with only 18% reaching Level 3 or higher, which enables data linkage between processes or predictive analytics." He added that the adoption of smart manufacturing and AI-based process technology is generally slow due to regulatory uncertainty and a lack of specialized personnel.To reduce the gap with global standards, Chun proposed establishing a national manufacturing and quality innovation roadmap and providing institutional support. He proposed several measures, including ▲Introducing manufacturing innovation incentives (tax benefits, subsidies, drug price preference) ▲Expanding field-based specialized workforce training programs ▲Establishing smart factory guidelines ▲Expanding public-private cooperation and sharing successful cases ▲Improving regulations for continuous manufacturing and Quality by Design (QbD).Chun added, "Securing global-level manufacturing competitiveness cannot be achieved solely through the efforts of individual companies." He emphasized, "As the industry aims to invest in AI and digital-based quality management, the government must promptly establish regulatory guidance and eliminate investment uncertainty for manufacturing innovation to realize."Hyun Soo Cho, a Manager at the Korea Biomedicine Industry Association, said that the CDMO industry needs to be developed by the government as a strategic industry. Cho noted, "The global biopharmaceutical market is being reshaped around manufacturing and quality capability, granting strategic advantage to countries that secure production infrastructure," and asserted, "Since South Korea already possesses the foundation for becoming a global CDMO hub, with the world's largest single production cluster (2.14 million liters) and large-scale facility capacity projected by 2030."Cho projected that the recently enacted 'Special Act on Regulatory Support for Biopharmaceutical Contract Development and Manufacturing Organizations (CDMO Special Act)' will be a turning point for the Korean CDMO industry. The CDMO Special Act, passed by the National Assembly on December 2, is a state-level industrial promotion law designed to help biopharmaceutical CDMOs meet global manufacturing/quality requirements and support exports/regulatory responses. Cho explained that institutional mechanisms such as the introduction of an export manufacturing registration system, quality management conformity certification, and raw material certification will further enhance the competitiveness of the domestic CDMO industry.Additionally, Cho emphasized the need to expand large-scale production bases, secure specialized personnel, and foster technology-focused CDMOs focused on next-generation modalities such as mRNA, cell/gene therapies, and ADCs. He stated, "It is urgent to expand the ecosystem to support successful companies and cultivate mid-sized and venture CDMOs as growth engines," and concluded, "For Korea to become a global production hub, support for process development, financial/tax incentives, and strengthening global certification capabilities must be pursued simultaneously."At the forum, policy directions for manufacturing, quality innovation, and enhancing global competitiveness were also discussed. In particular, the industry called for expanding the ecosystem, including the regular sharing of best practices in manufacturing/quality, customized professional workforce training, and matching support for joint development and process development between mid-sized/venture CDMOs and global companies.Yong Ki Park pointed out, "If the CDMO Special Act is the starting point for institutional foundation, then effective tax support and reform of the liability structure must follow in the subsidiary regulations," and criticized the current structure, which unilaterally transfers domestic liability to the manufacturer, saying it "does not align with global standards and could weaken competitiveness." Park stressed, "The establishment of a dedicated center for CDMO workforce training is urgent." He emphasized, "A national infrastructure must be established to systematically cultivate experts in GMP, process engineering, and quality who can be immediately deployed in the field, enabling the country to maintain its status as a global production hub."Yoon Hee Choi also argued that policy establishment is essential to strengthen R&D capabilities and transition to smart manufacturing. Choi advised, "A bold national strategy is required, including support for digital and AI-based process innovation in mid-sized and small companies, expansion of tax/subsidy incentives for nurturing technology-specialized CDMOs, and investment in professional infrastructure." Cho recommended, "It is necessary to support the growth of mid-sized and small CDMOs capable of customized biomanufacturing, such as cell and gene therapies, and establish collaboration platforms for process development and building track records between large corporations and ventures."
- Policy
- ‘Gov’t investment and incentives needed to resolve drug shortages’
- by Jung, Heung-Jun Dec 15, 2025 09:16am
- Experts stressed that resolving shortages of essential medicines requires financial investment and policies like drug price incentives on the government’s part.They also argued that reliance solely on the private sector is insufficient, calling for the establishment of public manufacturing infrastructure and proactive cooperation frameworks in areas where dependence on overseas supply is unavoidable.Park Silvia, Research Fellow, Korea Institute for Health and Social AffairsOn the 12th, Park Silvia, a researcher at the Korea Institute for Health and Social Affairs, emphasized the need for policy support to enhance drug supply stability at the Korean Academy of Social and Administrative Pharmacy academic conference.She explained that since the COVID-19 pandemic, countries like the US and Europe are also preparing countermeasures to address pharmaceutical supply disruptions.The US is increasing domestic manufacturing through executive orders and taking measures to ensure excess capacity within its domestic supply chain. To expand domestic production, it provides financial support incentives and has established an evaluation system that rewards excellent manufacturers.This year, regulatory barriers have been lowered to facilitate the establishment of domestic pharmaceutical manufacturing facilities and to shorten approval timelines.Europe is also pursuing strategic projects to strengthen manufacturing capabilities. It has also strengthened supply planning and reporting obligations for license holders and expanded joint purchasing among member states to bridge gaps in access to essential medicines.Park noted that Korea is also seeing a rising number of reports related to drug supply disruptions and shortages, underscoring the urgency of policy responses.Park stated, “Strengthening the essential medicine manufacturing and supply chain has clear limitations when relying solely on corporate willpower. Financial support such as subsidies, grants, low-interest loans, investments, and tax incentives is necessary for developing manufacturing technology and establishing facilities.”She further proposed, “It is necessary to identify priority medicines for focused support and management, centered on national essential medicines. Priorities must be assigned to derive strategies. Additionally, manufacturers' supply risk management plans must be established and their implementation managed.”Park added, "Preferential drug pricing is needed to maintain domestic manufacturing of essential medicines, including APIs. Essential medicines for which domestic manufacturing and diversification are strategically pursued should receive preferential treatment in public procurement.“Finally, Park stressed the importance of the public role, stating, ”Relying solely on private pharmaceutical companies to supply all essential medicines has limitations. Building a public-centered pharmaceutical manufacturing infrastructure is also necessary. Furthermore, in areas where dependence on the global supply chain is unavoidable, a system that ensure active cooperation and solidarity is needed."
- Policy
- Discussions on RWE utilization in reimb decisions
- by Jung, Heung-Jun Dec 15, 2025 09:16am
- Guidelines for using Real World Evidence (RWE) in both the initial listing and subsequent reassessment of drug reimbursement status are expected to be announced in South Korea next year.The research commissioned by the Health Insurance Review and Assessment Service (HIRA) is nearing completion, and the guidelines will be published after gathering industry feedback.However, to use the guidelines for drug reimbursement, significant challenges remain related to securing the reliability of Real-World Data (RWD) and RWE. Furthermore, pushback is anticipated from pharmaceutical companies that are expressing concerns about the burden of generating RWE.On the afternoon of December 12, HIRA officials, the National Health Insurance Service (NHIS), and pharmaceutical companies engaged in a lively debate on the use of RWE for reimbursement decisions at the Korean Society of Social Pharmacy conference.Ra-Won Kang, Department Head of Drug Performance Assessment Management at HIRA.The HIRA announced its plan to publicize the guidelines soon following the completion of research on the use of RWE.Ra-Won Kang, Department Head of Drug Performance Assessment Management at HIRA, stated that the research on RWE utilization is nearing completion, and the guidelines will be published soon.Kang said, "Many major international organizations utilize RWE. In countries like Canada and the UK, RWE is largely used as a crucial basis for decision-making during both initial listing and post-listing reassessment of evidence."Kang emphasized, "The current challenge is how to ensure the reliability of the collection of RWD for the generation of RWE. Research into international cases shows that countries use guidelines tailored to their own circumstances for quality control. HIRA has also developed RWE guidelines research, and once it concludes this year, we plan to publish the guidelines next year after gathering input from stakeholders."Se-Rim Oh, Department Head of Negotiation and Post-Management at the NHIS.The statement conveys the message that RWE utilization is the government’s response to resolving uncertainties about increasing drugs subject to economic evaluation exemptions.Se-Rim Oh, Department Head of Negotiation and Post-Management at the NHIS, explained that the government's interest in utilizing RWE stems from concerns about resolving uncertainties amid the increasing number of drugs subject to economic evaluation exemptions.Oh stated, "Some responsibility lies with pharmaceutical companies. Many drugs have entered the market through the economic evaluation exemption route, even though an economic evaluation could have been conducted. Half of the risk-sharing agreement drugs approved this year and last year entered through this exemption," noting that this increases the uncertainty regarding cost-effectiveness. Oh stated, "RWE should be used if necessary. However, generating data can be expensive and difficult. There are concerns that predictability and acceptability might decrease," and added, "If necessary, establishing a legal basis and creating a government-wide registry would be appropriate." She also proposed that expanding performance-based refund agreements conducted by the NHIS and utilizing periodic performance reassessment data could be viable methods.Industry "RWD data have not been standardized…overseas data should also be allowed"The pharmaceutical industry expressed concern that the RWE guidelines could inadvertently become an additional layer of regulation, similar to clinical trials.Companies have complained about the heavy burden of RWE generation, particularly since RWD standardization has not yet been achieved. They strongly advocated for the recognition of international RWE data.Junghyun Na, Daiichi Sankyo Korea Head, stated, "First, the scope of application must be clearly defined. Also, the data in the field are varied and not standardized. Companies will inevitably face a substantial burden." Na emphasized that infrastructure, such as public platforms, must be built before implementation.Furthermore, companies stressed that if the process by which RWE will be reflected in reimbursement and pricing decisions is not predictable, the investment risk for companies would be significant.Jongryun An, Senior Director at Janssen Korea, explained, "Drugs entering through the economic evaluation exemption are reviewed without consideration for cost-effectiveness, but they typically enter at the lowest possible price. Furthermore, they are subject to total expenditure limits." An argued that measures to control high costs are already in place.An also raised the issue that collecting sufficient domestic data for rare and severe disease treatments in South Korea is challenging due to the small patient pool. An requested, "Please be flexible in accepting global RWE data if it was used in the process of obtaining approval or reimbursement in other countries."Additionally, An argued that the guidelines should be flexible, stating, "Overly detailed or strict guidelines could lead to a situation where no decisions can be made."
- Policy
- Bambec to withdraw from Korean mkt after 31yrs
- by Lee, Tak-Sun Dec 12, 2025 07:54am
- Bambec 10mg (bambuterol hydrochloride), used for asthma, is being withdrawn from the domestic market. This is a strategic withdrawal made by a multinational pharmaceutical company in response to declining competitiveness.Yuhan Corporation reported the discontinuation to the MFDS on December 10, noting that the final supply date was October 31.The company stated, “The discontinuation occurred following AstraZeneca Korea’s decision to cease sales, which led to the termination of the contract manufacturing agreement.”Approved in August 1994, Bambec had been sold in Korea for more than three decades. It is a long-acting β-adrenoceptor agonist (LABA) used for bronchial asthma, chronic bronchitis with bronchospasm, pulmonary emphysema, and other respiratory diseases.In recent years, combination therapies have dominated asthma and COPD treatment, reducing the market need for LABA monotherapy products and weakening their market competitiveness. According to UBIST data, Bambec's outpatient prescription sales reached KRW 1.4 billion in 2024.AstraZeneca sells other respiratory drugs in Korea besides Bambec, including Daxas, Pulmicort, and Symbicort. Compared to other products, Bambec’s sales performance is relatively low, and this relatively small market performance likely contributed to the global headquarters’ strategic decision to discontinue sales.Fortunately, generic versions remain available, so the discontinuation of the original drug is unlikely to create a significant gap. Generic versions with the same ingredients and formulation include Chong Kun Dang’s Asterol, Medica Korea’s Bambi, and Ildong Pharmaceutical's Bambutol.
- Company
- Cardiomyopathy indication added to Amvuttra
- by Eo, Yun-Ho Dec 12, 2025 07:54am
- The RNAi therapy Amvuttra may be additionally approved to be used for ATTR-CM.The Ministry of Food and Drug Safety (MFDS) is currently reviewing expanding the indication for Amvuttra (vutirisiran), developed by Alnylam and introduced by Medison Pharma Korea, to treat transthyretin amyloid cardiomyopathy (ATTR-CM).Amvuttra was first approved in Korea in November 2023 as a treatment for transthyretin familial amyloid polyneuropathy (hATTR-PN).Administered as a single subcutaneous injection every 3 months, Amvuttra targets and silences specific messenger RNA, blocking the production of both wild-type and hereditary transthyretin (TTR).The drug’s efficacy in ATTR-CM was demonstrated through the Phase III HELIOS-B study.Designed as a randomized, double-blind, placebo-controlled, multicenter, global clinical trial, the study included diverse patients, including those previously treated with standard therapies such as Vyndaqel (tafamidis) and sodium-glucose cotransporter-2 (SGLT-2) inhibitors.Study results showed that Amvuttra significantly reduced the risk of all-cause mortality or recurrent cardiovascular events by 28% compared to placebo in patients with ATTR-CM. Furthermore, the risk of all-cause mortality or recurrent cardiovascular events was reduced by 33% in the group receiving only Amvuttra without Vyndaqel.ATTR-CM is a systemic protein deposition disorder caused by structural instability of transthyretin (TTR).TTR is a tetrameric transport protein primarily synthesized in the liver, responsible for stabilizing and transporting thyroid hormones and vitamin A. However, when genetic mutations or age-related changes destabilize the protein, the tetramer dissociates into monomers. These monomers then misfold and convert into insoluble amyloid fibers with a β-sheet structure. The accumulated amyloid fibers deposit in various organs, causing structural damage and functional decline.Meanwhile, Medison Pharma is currently working toward reimbursement listing for Amvuttra’s hATTR-PN indication. The drug recently passed the Drug Reimbursement Evaluation Committee review on December 4 and is now set to enter pricing negotiations with the National Health Insurance Service.