- LOGIN
- MemberShip
- 2025-12-17 19:27:37
- Policy
- 'Photos' to be excluded from files for negotiated drugs
- by Jung, Heung-Jun Nov 19, 2025 06:08am
- "Some companies submitted product photos and test certificates, but their production records were not found the following year. This must not happen." The National Health Insurance Service (NHIS) made this statement during a recent drug price consultation session with pharmaceutical industry stakeholders, announcing future institutional changes. According to industry sources on November 18, the NHIS is gathering opinions on changes to the documentation required for negotiated drugs and the scope of disclosure for Risk-Sharing Agreement (RSA) drugs, until November 21. The main point of the changes to the negotiated drug documentation is to replace product photos with the Manufacturing Instruction Record. The goal is to prevent instances such as the submission of a semi-finished product test certificate or the forgery of a finished product test certificate when no finished product inventory is actually available for shipment. Consequently, product photos will not be required unless requested. The primary objective is to substitute these with the Manufacturing Instruction Record to secure credibility. The NHIS plans to conduct an internal review in December and apply these changes starting with the main negotiations for negotiated drugs in January next year. The NHIS is also pursuing the disclosure of information regarding RSA drugs. The NHIS is also seeking opinions on the scope of information to be disclosed, including the drug name and type, within the same deadline. Some in the National Assembly have argued that related information must be disclosed to ensure the transparent operation of the RSA system. The NHIS is seeking opinions on two options: Option 1, which discloses only the drug name, and Option 2, which discloses both the drug name and the RSA type. The NHIS is also gathering general opinions on the overall disclosure of RSA drug information. The NHIS plans to disclose drug name and the contractually set refund rate to subsequent reimbursement applicants. An NHIS official previously stated, "We believe it is right to disclose the refund rate information for RSA drugs. We will ensure that this information is provided upon request when a reimbursement application is submitted to HIRA. However, the requesting applicant must sign a non-disclosure agreement."
- Product
- Discussions on expanding emergency OTC drugs gain momentum
- by Kang, Hye-Kyung Nov 19, 2025 06:08am
- As civic groups continue to demand the expansion of emergency OTC medicines available in convenience stores, the National Assembly will hold a policy forum on the issue on the 28th. According to industry sources on the 18th, Rep. Ji-a Han of the People Power Party, a member of the National Assembly's Health and Welfare Committee, will host a ‘Policy Discussion on Convenience Store Over-the-Counter Medicines to Promote Public Health’ on the 28th. Participants will include Soon-hyuk Kang, Director of the Division of Pharmaceutical Policy at the Ministry of Health and Welfare; Hye-ri Ahn, Secretary-General of the Consumer Network for Public Interest; and Chun-bae Park, Vice President of the Korean Pharmaceutical Association, who will represent the positions of their respective groups – the government, consumer groups, and the pharmacy community. Civil society groups advocating for expanding the list of over-the-counter emergency medicines and the convenience store industry are also showing great interest in the upcoming forum. Under a relevant system introduced in November 2012, up to 20 types of emergency OTC medicines may be designated for sale at convenience stores. However, civil society groups and the industry claim that only 13 items are actually designated as OTC medicines, and even among those, 2 items are currently suspended from sale. Although the system was initially designed to re-evaluate the designated items every 3 years, the Emergency OTC Medicines Review Committee has not convened since 2018, reportedly due to opposition from the Korean Pharmaceutical Association. At this year’s National Assembly audit, Rep. Han already highlighted both the need to expand the list and the prolonged delay in convening the review committee. Han argued that the system should be improved by easing the requirement for 24-hour year-round sales, removing the cap of 20 designated items, and establishing a solid legal basis for committee operations to prevent conflict between professional groups. In response, Minister of Health and Welfare Eun-kyeong Jeong stated, “The emergency OTC medicine system has been in place for over 10 years and must be updated to reflect current circumstances. We should first adjust the list of items or discontinue products that are no longer sold. Also, since there are no 24-hour convenience stores in areas without pharmacies, the 24-hour operation restrictions should be relaxed. We are preparing a comprehensive plan and are also consulting with organizations like the Korean Pharmaceutical Association.” The KPA maintains that issues in pharmacy-desert regions can be resolved through the “Special Locations for Pharmaceutical Handling” notice. This policy allows designated individuals to dispense or provide medications in locations with limited access to pharmacies—such as ships, aircraft, remote islands, or military bases.
- Policy
- World’s first dutasteride+tadalafil combo to launch in Dec
- by Lee, Tak-Sun Nov 19, 2025 06:07am
- The world’s first dutasteride and tadalafil combination approved for benign prostatic hyperplasia (BPH) is expected to be released next month as a non-reimbursed product. This effectively confirms that the companies failed to secure reimbursement for their respective products. According to industry sources on the 17th, the dutasteride+tadalafil fixed-dose combination will begin sales next month at an expected price of KRW 1,300. Four products from four companies received approval: Dong-A ST's Dutana Tab 0.5/5mg, Shinpoong Pharm’s Avocial Tab 0.5/5mg, Dongkook Pharmaceutical's Uresco Tab 0.5/5mg, and DongKoo Bio&Pharma's Uroguard Tab 0.5/5mg. The four drugs were approved on January 23rd through joint development. The approved indication is the treatment of moderate to severe symptoms of benign prostatic hyperplasia. Dongkook Pharmaceutical is the manufacturer. These companies received approval in January and have been pursuing reimbursement listing. However, because tadalafil is a non-reimbursed ingredient, the combination could not be priced under standard calculations and would have had to enter the new drug reimbursement track, which requires substantial data submission and a prolonged evaluation period. Ultimately, manufacturers agreed to proceed with a non-reimbursed market launch. Some companies are informing distributors and retailers of their new product launch plans for December. The KRW 1,300 price is viewed as reasonable. Currently, the maximum price for dutasteride tablets 0.5mg is KRW 709. Given that the lowest price for tadalafil 5mg is around KRW 700, the combination drug’s price is lower than the sum of the two components. Still, since most BPH treatments in Korea are reimbursed, it remains uncertain how much demand there will be for a non-reimbursed option. Industry observers note that manufacturers may lean toward highlighting tadalafil’s erectile dysfunction benefits as a marketing angle.
- Opinion
- [Reporter's View] New obesity drug sparks dilemma
- by Son, Hyung Min Nov 18, 2025 06:13am
- Following approval of an expanded indication for Wegovy (semaglutide), a Glucagon-like Peptide-1 (GLP-1)- based obesity treatment, to include adolescents aged 12 and older in South Korea, the discussion surrounding pediatric and adolescent obesity has once again come into the spotlight. While the global market already defines pediatric obesity as a medical disease requiring early intervention, South Korea is still lagging in both social awareness and institutional support. Obesity cannot be explained merely as an aesthetic or lifestyle issue. In particular, obesity during the growth period is highly likely to persist into adulthood. It serves as the starting point for chronic diseases such as Type 2 diabetes, hypertension, non-alcoholic fatty liver disease, and cardiovascular disease. The Obesity Fact Sheet 2025, published by the Korean Society for the Study of Obesity (KSSO), demonstrates this reality. Although the obesity rate in children and adolescents has slightly decreased in the last five years, approximately three out of 10 are still classified as obese. The obesity rate peaked at age 14 for boys and after age 16 for girls. It was also confirmed that the probability of obesity in children is five times higher if their parents are in obesity class 2 or higher. The problem is that it does not end with simple weight gain. Adult chronic diseases such as Type 2 diabetes, hypertension, and liver disease are actually increasing among obese and overweight children and adolescents. The pace of change has accelerated to the point where Type 2 diabetes in adolescents is no longer considered a rare condition in modern clinical practice. Experts note that obesity during the growth period is not a short-term problem; instead, the timing of exposure determines the severity of the disease. Obesity starting early in life has a prolonged duration, increasing the risk of complications and potentially exploding lifelong management costs and medical burden. The current situation is different from the era when obesity was dismissed as merely an aesthetic concern. Despite this severe situation, the treatment environment in South Korea remains stagnant. While innovative treatments like GLP-1 drugs have emerged, the perception that obesity is a treatable disease is spreading among adult patients. Conversely, in the U.S., major new obesity drugs have already been approved for adolescents and are actively used in clinical practice. Although South Korea tends to tighten regulations due to concerns over misuse and abuse, some critics suggest that patients in need are being deprived of the right to choose treatment. Obesity cannot be explained solely by willpower or lifestyle choices. Appetite is a biological system involving the pituitary-hypothalamic axis, adipose tissue, and various hormones. It is structurally harder for growing adolescents to control their appetite than it is for adults. Without addressing the underlying physiological factors, lifestyle modifications alone will inevitably face apparent limitations. However, drug therapy is not an answer for everyone. The effectiveness of obesity treatments is maximized when combined with healthy lifestyle modifications. Especially during the growth period, lifestyle education, continuous counseling, and long-term management are essential. However, experts are deeply concerned that delaying drug treatment for adolescents who already show signs of complications, such as hypertension, elevated liver enzymes, or pre-diabetes, will exponentially increase the future disease burden. The core of the problem is the social frame through which obesity is viewed. Many still interpret obesity as a failure of lifestyle or lack of willpower, and the perception exists that obesity during childhood and adolescence will 'naturally resolve itself as individuals grow.' However, obesity during the growth period cannot be solved by time. Delayed early intervention exponentially increases long-term health risks and places an even greater medical and social burden on the patient than adult obesity. While the perception of obesity as a disease is slowly being built, regulations remain overly strict, and access to treatment is restricted. While preventing misuse is critical, a situation where even patients in need cannot access medication is another form of neglect. What is needed now is not more regulation, but balance. Ensuring safety and preventing misuse must be fundamental policy goals, but those principles should not stand in the way of timely treatment. Obesity during childhood and adolescence is a key turning point that determines lifelong health. The treatments are available. The remaining challenge is for social awareness and institutional support to catch up so that these treatments can reach the patients who need them.
- InterView
- Anzupgo to address the unmet needs in chronic hand eczema
- by Eo, Yun-Ho Nov 18, 2025 06:13am
- Professor Sonja Molin, Division of Dermatology, Department of Medicine at Queen Since their emergence, JAK inhibitors have rapidly expanded their therapeutic footprint. Initially introduced as the first oral option in rheumatoid arthritis, a field previously reliant solely on injections, JAK inhibitors have since taken a pivotal role across the spectrum of autoimmune diseases, including ankylosing spondylitis, psoriatic arthritis, atopic dermatitis, and Crohn’s disease. In this context, JAK inhibitors are now breaking yet another barrier in disease management by shedding even the notion of being an ‘oral formulation’ and entering the market as topical ointment formulations, ushering in a new paradigm shift. In September, the Ministry of Food and Drug Safety granted marketing authorization for Anzupgo (delgocitinib), a treatment for chronic hand eczema (CHE). Specifically, Anzupgo is indicated for the topical treatment of“moderate-to-severe chronic hand eczema in adults who have an inadequate response to, or for whom treatment with topical corticosteroids is not advisable.” As a non-steroidal, topical, pan-JAK inhibitor, Anzupgo inhibits the activation of the JAK-STAT cellular signaling pathway, known to play a key role in the manifestation of hand eczema. Until now, treatment options for chronic hand eczema have been limited, with strong topical corticosteroids commonly used as first-line therapy. However, its long-term use carries risks of various side effects, including skin barrier damage, skin atrophy, and telangiectasia. Dailypharm met with Dr. Sonja Molin, Professor of Dermatology at Queen's University, to discuss the clinical value and therapeutic potential of Anzupgo, the first topical JAK inhibitor for CHE. Anzupgo cream-What is chronic hand eczema, and what is its prevalence? CHE is one of the most common skin conditions affecting the hands, and a large proportion of cases progress to chronic disease. CHE is known to affect approximately 1 in 10 adults worldwide. This disease causes significant functional, occupational, and psychological burdens, significantly reducing patients' quality of life. Approximately 70% of patients with severe CHE experience difficulties performing daily activities, which directly impacts their employment and income. As a result, early diagnosis and appropriate treatment are paramount to effectively reduce the disease burden for these patients. Therefore, raising awareness about CHE and expanding active treatment approaches are urgently needed. Many patients only visit general practitioners rather than dermatologists, meaning a significant number of cases are not fully reflected in official statistics. Consequently, the true prevalence of hand eczema is likely much higher than current estimates. -Chronic hand eczema is known to be morphologically diverse. Which subtypes primarily occur? CHE is a heterogeneous disorder influenced by multiple factors and manifests in various forms. According to the 2022 European guidelines, CHE is categorized into etiological subtypes and clinical subtypes. In practice, overlapping subtypes are quite common, with irritant contact dermatitis and allergic contact dermatitis being common causes. Clinical subtypes like bullous or hyperkeratotic hand eczema also require careful treatment. -Does Anzupgo show efficacy across all CHE subtypes? Anzupgo is approved for moderate to severe chronic hand eczema in adults who do not respond to or are not suitable for topical steroid therapy. Results from the Phase III clinical trial supporting its approval demonstrated that delgocitinib cream showed broad therapeutic responsiveness across all CHE subtypes. Mechanistically, Anzupgo not only provides anti-inflammatory effects but also helps restore the damaged skin barrier, making it suitable for treating irritant, allergic, vesicular, and hyperkeratotic subtypes. Clinically, patients with thick, severe hyperkeratotic lesions may exhibit slower treatment response. However, CHE is a chronic condition requiring long-term management, so continued treatment should be considered with expectations for gradual improvement. -What limitations exist with previous CHE treatments, and how does Anzupgo differ? As a dermatologist, I have long felt that existing treatment options for CHE are inadequate. For example, topical corticosteroids are internationally recommended as first-line therapy, but repeated long-term use often leads to diminished efficacy and local adverse effects, which are clear limitations. Alitretinoin is the only oral medication approved specifically for CHE and is effective for symptom control. However, concerns regarding teratogenicity restrict its use in women of childbearing potential, and routine lab monitoring is required, limiting its broader use. This unmet need means the disease burden associated with patients' symptoms remains a challenge that needs to be addressed. Therefore, the arrival of Anzupgo, a topical therapy with a novel mechanism and clinically proven efficacy and safety, is extremely welcome. - You have real-world experience prescribing Anzupgo. How do clinical outcomes compare with trial data? Having participated in the DELTA study, the pivotal clinical trial for Anzupgo, I was therefore already familiar with its efficacy and safety profile. As a dermatologist treating patients with CHE, my primary concern was whether the efficacy and safety profile demonstrated in clinical trials would translate identically to real-world patient outcomes. In conclusion, Anzupgo met these expectations and, in some cases, demonstrated even superior efficacy in real-world practice. Notably, it showed excellent responsiveness in the bullous subtype of CHE, where response to existing medications was often poor. Furthermore, the results of the DELTA FORCE study, which directly compared Anzupgo with the oral agent alitretinoin, are also impressive. It was surprising that delgocitinib cream demonstrated statistically significant clinical results compared to alitretinoin in the primary endpoint (change in HECSI score at Week 12 from baseline) and all other efficacy parameters. -When should patients previously treated with topical steroids consider switching to Anzupgo? If the response to topical corticosteroids is inadequate or relapses are frequent, the physician should consider other treatment options as early as possible. Since its approval in Germany last September, the use of Anzupgo has steadily increased. Awareness of delgocitinib cream is gradually growing among both healthcare professionals and patients, and overall treatment satisfaction is high.
- Company
- Will the 53.55% generic drug pricing system be overhauled?
- by Kim, Jin-Gu Nov 18, 2025 06:12am
- The Korean government is moving toward a full-scale overhaul of the national drug pricing system Structural overhauls of the overall domestic drug pricing system are materializing, including adjustments to the generic drug pricing calculation rate, reform of the tiered pricing system, consolidation of post-marketing control systems, expansion of risk-sharing agreement and dual pricing systems, and introduction of R&D investment-linked drug pricing premiums. According to industry sources on the 18th, the Ministry of Health and Welfare is scheduled to attend the Korea Pharmaceutical and Bio-Pharma Manufacturers Association’s board meeting this afternoon to explain the direction of the drug pricing system reform. It is understood that Lee Jung-kyu, Director-General of the Bureau of Health Insurance Policy, will personally explain the reform's purpose and direction and request the pharmaceutical industry's participation. The Ministry has been continuously discussing the reform of the drug pricing system. Discussions have accelerated, particularly since the inauguration of the Lee Jae-myung administration. The outline has been shaped to simplify an overly complex system, making it more predictable, while simultaneously encouraging R&D investment by domestic pharmaceutical and biotech companies. A draft of the reform plan is reportedly already prepared. Following the ministry's briefing to the KPBMA, the discussions on drug pricing system reform, which have been progressing behind the scenes, are expected to move into the official phase. The focal point of interest is the generic drug price calculation rate. Lowering the current 53.55% rate is understood to be one of the core elements of the reform plan. Under the current system, the generic drug price is set at 59.5% of the original drug's highest price for the first year after initial listing. From the second year onward, the price is maintained at a reduced rate of 53.55%. To qualify for the 53.55% rate, both conditions must be met - ‘conducting its own bioequivalence test’ and ‘using a registered Drug Master File registered API.’ Meeting only one condition results in an additional 15% reduction (45.52% of the original price). Failing to meet both conditions leads to a further 15% reduction (38.69% of the original price). The government believes the current 53.55% benchmark is excessively high. Although the exact adjustment level has not been disclosed, industry insiders expect a considerably lower figure, potentially around 40%. An industry official stated, “If the discussion were merely about lowering the 53.55% calculation rate to 50%, the reform talks wouldn't have even started. The consensus is that the final figure will be below 50%.” The tiered generic pricing system is also expected to face revisions. Currently, the first 20 generic products maintain the 53.55% price level, and after the 20th, the price is reduced by 15% sequentially. The 21st generic is priced at 85% of the lowest price among the first 20 products, and the 22nd generic is priced at 85% of the 21st generic, and so on. In this regard, the Ministry of Health and Welfare considers the ‘20-product’ range as excessively broad. It is reported that a plan to reduce this to around 10 products is under review. However, it is understood that discussions are also underway to moderate the structure where prices drop by 15% after the 20th product, meaning fewer generics per tier, but smaller price drops per step. A major consolidation of post-management regulations is also underway. Current systems, including the ▲ Actual Transaction Price (ATP) reduction, ▲ reassessment of reimbursement adequacy, and ▲ the Price-Volume Agreement system, run simultaneously. The government has also previously considered introducing an external reference pricing-based reevaluation. The Ministry of Health and Welfare plans to consolidate these systems to enhance predictability. To this end, the Ministry commissioned a study titled “Integrated Framework for Post-Management of Drug Pricing” to the Daegu Catholic University Industry-Academic Cooperation Foundation in March this year. Once the results are released at the end of the year, related discussions are expected to accelerate. The drug price premium system is also likely to undergo a significant overhaul. Given persistent criticism that the current premium system is overly complex, the government is considering a method to determine drug price premiums and preferential treatment based on the R&D investment ratio of pharmaceutical and biotech companies. A pharmaceutical industry insider stated, “It is clear that the government intends to overhaul the multi-product, generic-centered market structure. Based on the judgment that the system has become excessively complex due to additions made as needed over time, discussions are proceeding toward streamlining it within a broad framework to enhance predictability. The government intends to strongly convey the message that growth is no longer feasible solely through a generic-centered market.” A government-ruling party official explained, “The focus of this reform is not on cutting healthcare spending. The goal is to create an environment where pharmaceutical innovation can function properly. We are reviewing options such as linking pricing premiums to the company’s R&D investment ratio. The intent is to make R&D investment a key determinant of reward.”
- Company
- 'True innovation requires guaranteed access to new drugs'
- by Son, Hyung Min Nov 18, 2025 06:09am
- A recent survey on perceptions of pharmaceutical innovation showed that the vast majority of Koreans believe innovation should be assessed not only by clinical efficacy but also by whether patients can realistically access and use the treatment. The survey, commissioned by global pharmaceutical company BeOne Medicines and conducted by research firm Embrain from August to October 2025, aimed to objectively understand public perceptions of new drug accessibility and the need for improvement. A total of 1,000 Korean adults aged 20 and older participated, including more than 200 cancer or severe-disease patients and caregivers. Among respondents, 69.5% believed they or a family member could face cancer or a severe disease, and a similar proportion, 69.7%, replied that “accessing new drugs would be difficult.” The biggest barriers were financial burden (54.2%) and lack of information (52.2%). Older respondents perceived the lack of information about new drugs as a greater problem. Among respondents who had personally experienced or had a family member with cancer or a serious illness, 47% considered treatment with new drugs, but 74% of those reported difficulties accessing such treatments. 84% of respondents stated that ensuring accessibility after new drug development is essential for innovation, and 82.7% agreed that if a drug is inaccessible due to cost, it cannot be considered innovative. This indicates that the majority of Koreans perceive pharmaceutical innovation not merely as scientific progress but also as encompassing broader social value. The government (89%) was identified as the most important entity for improving new drug accessibility, followed by healthcare professionals (83.5%) and pharmaceutical companies (64.2%). 70.8% of respondents felt that the role of certain stakeholders was being over-emphasized in the current system, while 87.2% agreed that collaboration among all stakeholders is necessary. Regarding the roles expected of each entity to improve new drug accessibility, the most common response (receiving positive responses from over 80% of respondents) was that the government should secure flexibility in new drug price evaluations and relax/expand reimbursement criteria to broaden coverage. Healthcare professionals needed to provide patients with information on new drugs and disease education, and to assist in selecting optimal treatments through sufficient communication with patients. Pharmaceutical companies needed to contribute to improved accessibility by expanding clinical trials and engaging in reasonable price negotiations. Patient groups needed to lead efforts to improve awareness through sharing patient experiences and advocating for healthcare policy improvements. In-depth interviews conducted to gain deeper insights from the quantitative survey results and derive practical improvement measures revealed that experts from various sectors, government, pharmaceutical industry, patient groups, and media, unanimously agreed that for the innovation of new drugs to truly reach patients, cooperation across systems, industry, and society as a whole must function together. The most urgent issue identified was structural reform of the pricing and reimbursement system. Experts noted that the system remains largely unchanged from 20 years ago and pointed to shortages of trained reviewers and delayed assessment timelines as major bottlenecks. There was broad consensus on the need for a shift toward a reimbursement structure prioritizing severe diseases, improved predictability in pricing evaluation procedures, and greater flexibility to accommodate patient-specific circumstances. Additionally, establishing a sustainable ecosystem for new drug supply was presented as a key task. Emphasis was placed on transparency of data during drug price negotiations, reasonable price negotiations, and responsible efforts by the pharmaceutical industry to prevent market withdrawal due to pricing issues. Lastly, patient-centered decision-making and strengthened public dialogue were deemed necessary. Experts pointed out that the rigid reimbursement criteria limit treatment choices for individual patients, proposing institutional mechanisms to bridge the gap between medical professionals' judgments and patient expectations. They also emphasized the importance of the media and civil society's role in consistently highlighting treatment access issues and fostering public discourse. Ji-yeon Lee, Team Leader of the Business Division at Embrain Research, who oversaw this survey, stated, “This survey confirmed that the public recognizes the innovation of new drugs not just as technological achievements, but as social value that actual patients can tangibly experience. It is time to ‘redefine innovation’ to include not only efficacy but also accessibility, and this public perception needs to be actively reflected in future policy and system improvement discussions.” Jihye Yang, CEO of BeOne Medicine Korea, remarked, “This survey confirms the public's recognition that innovation must be proven in patients' lives, not just in laboratories. The background enabling BeOne Medicine Korea to drive the rapid reimbursement introduction of Brukins and Tevimbra was internalizing clinical development and streamlining operational models. Innovation can reach patients when all stakeholders—the government, healthcare professionals, and patient groups—act and cooperate."
- Company
- ABL Bio shows successful technology transfer…Lilly invests
- by Cha, Jihyun Nov 18, 2025 06:08am
- ABL Bio, a company specializing in bispecific antibodies, successfully secured both a large-scale technology transfer agreement and a strategic investment from Eli Lilly, a global pharmaceutical company. These deals instantly provided the company with over KRW 80 billion in cash reserves. The consecutive deals proved the company's global technological competitiveness, causing the stock price to surge. ABL Bio's market capitalization approximately doubled in just three days, from the KRW 5 trillion range to the KRW 10 trillion range. According to the Korea Exchange (KRX) on November 16, ABL Bio's stock price closed at KRW 174,200, a 6.5% increase from the previous trading day. Earlier that day, ABL Bio briefly broke its 52-week high of KRW 195,500. ABL Bio's stock price surged for three consecutive trading days from the 12th to the 14th. Compared to the closing price of KRW 97,500 on the 11th, ABL Bio's stock price jumped approximately 78.7%. The market capitalization increased from KRW 5.3747 trillion on November 11 to KRW 9.6028 trillion on November 14, based on closing prices. During the day, the market cap peaked at KRW 10.77 trillion. The company's valuation nearly doubled in just three days. ABL Bio climbed to the 4th position by market capitalization on the KOSDAQ as of the November 14 closing price. ABL Bio Analysis suggests that ABL Bio's stock price surge was driven by its consecutive technology-transfer and strategic-investment agreements with Eli Lilly. ABL Bio previously signed a technology transfer and joint research and development (R&D) agreement with Eli Lilly on November 12 for its Blood-Brain Barrier (BBB) shuttle platform, 'Grabody-B'. The deal grants Eli Lilly exclusive worldwide rights to develop and commercialize multiple undisclosed target candidates, applying ABL Bio's Grabody-B platform to various modalities. The total value of the agreement is $2.602 billion (approximately KRW 3.8072 trillion). The non-refundable upfront payment is $40 million (KRW 58.5 billion), representing 1.5% of the total contract value. ABL Bio will receive up to $2.562 billion (approximately KRW 3.7487 trillion) in milestone payments, contingent on clinical, regulatory, and commercialization achievements. Royalties on net sales, if commercialization is successful, are separate. Notably, their agreement is a 'platform deal,' transferring comprehensive rights to develop multi-target therapies using the Grabody-B platform across various modalities, rather than a single candidate molecule. Since a global top-tier pharmaceutical company chose a platform that can be expanded across multiple disease areas and development methods, it is considered significant. ABL Bio previously signed an R&D agreement in April with the global pharmaceutical company GlaxoSmithKline (GSK) to develop neurodegenerative disease treatments using Grabody-B. That agreement was valued at £2.1401 billion (approximately KRW 4.1104 trillion). At the time, ABL Bio also entered a platform agreement with GSK to develop multi-target therapies using various modalities, including siRNA, ASO, and antibodies. This is significant as two global top-tier pharmaceutical companies, GSK and Eli Lilly, have chosen the ABL Bio platform. Summary of ABL Bio Such a platform's competitiveness is further confirmed by Eli Lilly's decision to make a direct equity investment. Two days after the technology transfer announcement, on November 14, ABL Bio announced a capital increase of 3rd-party share allocation worth $15 million (approximately KRW 22 billion) to Eli Lilly, specifically issuing 175,079 common shares at KRW 125,900 per share. The common shares issued in this transaction will be held in escrow at the Korea Securities Depository for one year. ABL Bio plans to invest the secured funds in R&D, specifically advancing Grabody-B and developing bispecific antibody-drug conjugates (ADCs). ABL Bio also announced its goal to use this investment to strengthen its new drug development capabilities and explore diverse opportunities for collaboration with Lilly for new drug development from a long-term perspective. This investment is particularly noteworthy because it is a strategic investment made separately from the technology transfer agreement, suggesting that Eli Lilly highly values the commercial potential and long-term partnership value of the Grabody-B platform. The fact that the same partner decided to make a consecutive strategic equity investment immediately after a technology transfer agreement is a rare event in the Korean biotech industry. The market views this as evidence that ABL Bio's BBB shuttle platform has been recognized as competitive on the international stage. Consequently, ABL Bio has immediately secured over KRW 80 billion in cash reserves, combining the KRW 58.5 billion upfront payment from the Grabody-B technology transfer with Lilly and the KRW 22 billion from the capital increase. Including the KRW 73.9 billion upfront payment from the GSK platform technology transfer in April, ABL Bio has secured over KRW 150 billion in cash reserves from global big pharma companies this year alone. ABL Bio is establishing a virtuous cycle by reinvesting funds generated from successive strategic investments and technology-transfer revenues into R&D. ABL Bio recently officially launched its U.S. local subsidiary, Neok Bio, to strengthen its global clinical system. Neok Bio is a company dedicated to the clinical development of bispecific ADC candidates. It will serve as a worldwide clinical hub, responsible for communication with U.S. regulatory authorities, conducting bispecific ADC clinical trials, and formulating development strategies.
- Policy
- First batch of drugs gain reimb through AEN pilot project
- by Jung, Heung-Jun Nov 17, 2025 06:11am
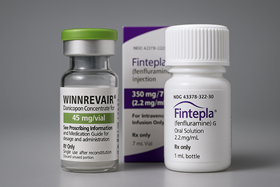
- With the first drugs under the Approval-Evaluation-Negotiation pilot program all securing reimbursement, the timing for the second batch of drugs currently under review to enter reimbursment listing is also approaching. Expectations are building for the possibility of their sequential insurance coverage as early as the first half of next year. With Bylvay Cap (odevixibat) gaining reimbursement coverage last month, all first-phase drugs under the Approval-Evaluation-Negotiation (AEN) project have now entered Korea’s reimbursement system, joining the previously covered Qarziba Inj (dinutuximab beta). The second batch of products under review are Winrevair (sotatercept) by MSD Korea for pulmonary arterial hypertension, Fintepla (fenfluramine) by UCB Korea for Dravet syndrome, and Limcato (anbal-cel) by the domestic company Curocell for large B-cell lymphoma. According to industry sources on the 16th, approximately 9 to 10 months have passed since the companies applied for reimbursement of these second-batch drugs. They are currently receiving cost-effectiveness reviews. Except for Winrevair, which received marketing authorization in July, the other two are also near approval. Among the three, Limcato was the first to apply for reimbursement in January, followed by Winrevair and Fintepla in February. Winrevair and Fintepla are being reviewed for cost-effectiveness, while Limcato is being reviewed for its clinical usefulness. Looking at the precedent set by the first batch of drugs that went through the reimbursement listing process, reimbursement for the second batch is expected early next year. As Qarziba was listed about 6 months after approval, if Winrevair, the only second batch drug approved as of now, follows a similar process, its reimbursement listing could occur as early as late this year or early next year. However, both Qarziba and Bylvay encountered hurdles at the Drug Reimbursement Evaluation Committee (DREC) stage, facing twists and turns before achieving reimbursement. Qarziba, which was approved by the Ministry of Food and Drug Safety (MFDS) in June last year, was initially deemed non-reimbursable at its first DREC review. It was subsequently granted for reimbursement in December last year after reapplying. It took approximately two years from Bylvay’s first reimbursement application in October 2023 to listing. Even at the point of its re-application last August, it had already been 14 months. This underscores the need to pass the DREC review without rejection and secure recognition of its reimbursement appropriateness, to achieve swift reimbursement listing. If a re-review process is required, reimbursement may be delayed beyond expectations. Meanwhile, the AEN linkage project is a pilot initiative aimed at enhancing access to high-priced treatments for severe diseases by shortening the period to listing of new drugs—which previously exceeded 300 days, including 120 days for MFDS (Ministry of Food and Drug Safety) approval, 150 days for HIRA (Health Insurance Review and Assessment Service) reimbursement evaluation, and 60 days for NHIS (National Health Insurance Service) drug price negotiations. During this year's National Assembly audit, the Ministry of Health and Welfare stated it would review plans to institutionalize the expedited listing system based on an analysis of the AEN pilot project's outcomes and feedback from the field.
- Company
- What's the outcome of the 2nd reimb attempt for 'Uplizna'?
- by Eo, Yun-Ho Nov 17, 2025 06:11am
- Attention has been drawn to whether 'Uplizna,' a new treatment for neuromyelitis optica spectrum disorders (NMOSD), will successfully gain insurance reimbursement listing at its second attempt. According to industry sources, Mitsubishi Tanabe Pharma Korea has accepted the conditional price that Health Insurance Review and Assessment Service (HIRA)'s Drug Reimbursement Evaluation Committee (DREC) suggested for Uplizna (inebilizumab), a treatment for adult patients with anti-aquaporin-4 (AQP-4) antibody-positive neuromyelitis optica spectrum disorder (NMOSD). The Ministry of Health and Welfare (MOHW) has ordered the National Health Insurance Service (NHIS) to initiate drug price negotiation. Therefore, the company is expected to begin discussions with the NHIS. The listing process for Uplizna was halted in October of last year during the drug price negotiation stage due to a supply issue. At that time, the company accepted a price below the amount evaluated by the HIRA's DREC and began drug price negotiations. However, a conclusion was not reached within the 60-day negotiation period. Although the National Health Insurance Service (NHIS) intended to enter into an extension negotiation, the renegotiation could not commence because the pharmaceutical company was unable to secure a domestic supply. NMOSD occurs when AQP4 autoantibodies, which are disease-specific markers produced by B cells, bind to the target antigen Aquaporin 4 (AQP4) on astrocytes in the central nervous system, thereby activating an immune response that leads to neurological damage. Uplizna is a CD-19-targeting humanized monoclonal antibody, developed using a novel mechanism, that prevents disease relapse by selectively binding to CD19, a B-cell-specific surface antigen, to deplete AQP4 antibody-producing B cells. Uplizna's safety and efficacy were demonstrated in the N-MOmentum clinical study, a monotherapy study conducted in 230 patients without concomitant immunosuppressants. The study results showed that 89% of patients who received Uplizna did not experience a relapse during the 197-day follow-up period, demonstrating a 77.3% reduction in relapse risk compared with the placebo group. The safety evaluation also showed a similar rate of adverse events to the placebo group.