- LOGIN
- MemberShip
- 2025-12-20 05:22:35
- Company
- Novo Nordisk, Kakao Health sign digital healthcare MOU
- by Cha, Jihyun Sep 04, 2025 06:09am
- Kakao Healthcare (CEO Hee Hwang) announced on the 3rd that it has signed a memorandum of understanding with the multinational pharmaceutical company, Novo Nordisk Korea (General Manager Kasper Roseeuw Poulsen) to build a digital healthcare ecosystem for obesity and diabetes patients. The signing ceremony, held on September 2 at Novo Nordisk Korea’s headquarters in Songpa-gu, Seoul, was attended by Kakao Healthcare CEO Hee Hwang and Novo Nordisk Korea CEO Kasper Rosseeu Poulsen. At the event, the two companies agreed to collaborate by utilizing various digital technologies to improve the treatment journey of obesity and diabetes patients, ultimately creating a sustainable treatment environment that can deliver better outcomes. This is the second collaboration between the two companies, following their first partnership in 2023. Under the agreement, they linked Kakao Healthcare’s AI-based health management app ‘Pasta’ with Novo Nordisk’s insulin FlexTouch pen and its smart cap ‘Mallya,’ providing a medication management solution for diabetes patients. The new partnership is significant in that it expands the scope of collaboration from diabetes to obesity, creating a comprehensive alliance. The companies plan to address the unmet medical needs of Korea’s rapidly growing obesity and diabetes populations by offering patient-centered digital solutions. Specifically, in the field of obesity, the partnership will focus on ▲developing a personalized digital support program, ▲providing solutions to improve treatment effectiveness and quality of life. and In particular, the partnership will integrate Novo Nordisk’s patient support program Novo Fit Care, which is offered to patients prescribed its obesity treatments, into the Pasta app. Through this, patients will be able to manage weight and other long-term health aspects in a comprehensive way. In the field of diabetes, the companies will jointly develop digital solutions aimed at improving disease awareness. Kasper Roseeuw Poulsen, General Manager of Novo Nordisk Korea, said, "Obesity and diabetes are chronic conditions which, if left untreated, can lead to serious comorbidities and impose a tremendous burden on individuals and society. With Novo Nordisk’s more than 100 years of dedication to diabetes and obesity, and Kakao Healthcare’s leadership in Korea’s digital healthcare sector, the partnership will further accelerate integrated innovation for patient support in Korea." Hee Hwang, CEO of Kakao Healthcare, said, "Kakao Healthcare has leveraged AI, big data, and other technologies to bring positive changes to patients’ health journeys. By combining this experience and technology with Novo Nordisk, a global leader in obesity and diabetes care, we aim to play a major role in expanding patient-centered digital healthcare on a global scale."
- Policy
- HIRA "Reimb application of Wegovy has not been filed"
- by Lee, Jeong-Hwan Sep 04, 2025 06:08am
- Product photo of Wegovy The Health Insurance Review & Assessment Service (HIRA) announced that it will conduct a fair and swift evaluation if an application for reimbursement is submitted for Novo Nordisk's popular obesity drug, Wegovy (semaglutide). HIRA clarified that since Wegovy's company has not yet applied for reimbursement, HIRA is yet at the stage of determining Wegovy's National Health Insurance reimbursement. HIRA stated recently after a recent inquiry from Rep. Kim Seon-min of the Cho Kuk Innovation Party regarding the reimbursement status of Wegovy. Rep. Kim had asked HIRA for its stance on a plan to transition the non-reimbursed prescription drug Wegovy to National Health Insurance coverage and management. Rep. Kim likely inquired about the management plan related to the significant prescription volume of Wegovy, which has garnered immense popularity since its launch in Korea. According to data from the Drug Utilization Review (DUR) system, approximately 400,000 prescriptions for Wegovy have been issued in the roughly eight months since its launch in October 2024, which translates to about 80,000 prescriptions per month. The non-reimbursed prescription price for Wegovy ranges from KRW 200,000 to 300,000 for the 0.25mg, 0.5mg, and 1.00mg doses, and over KRW 400,000 for the 1.70mg and 2.40mg doses. Related to this, Rep. Kim has raised the need for managing the side effects that arise from people who are not overweight or obese using these drugs for cosmetic purposes. The medical community has also pointed out that some doctors are prescribing Wegovy and other obesity drugs for cosmetic, rather than therapeutic, purposes. This practice, they argue, can lead to repeated prescriptions and illegal trading of excessively prescribed medicines on online platforms. To address these issues, the medical community is proposing that obesity treatments be brought under the National Health Insurance system, subjecting them to a public monitoring and management system. In response to these concerns, Rep. Kim asked HIRA for its plan to manage the side effects of Wegovy through reimbursement. However, HIRA's response was general and procedural. HIRA explained, "For a new drug to be listed for reimbursement, the pharmaceutical company must first apply for reimbursement to the Minister of Health and Welfare and the head of HIRA, along with the necessary documentation," and added, "Then, this is followed by HIRA will evaluate the drug's clinical utility and cost-effectiveness, followed by drug price negotiations with the National Health Insurance Service. Finally, a notification is issued by the Ministry of Health and Welfare." HIRA said, "Wegovy's manufacturer had not yet submitted a reimbursement application after its approval by the Ministry of Food and Drug Safety," and added, "If an application for this drug is submitted in the future, we will ensure that it is evaluated fairly and swiftly." Meanwhile, the decision on National Health Insurance reimbursement for new obesity drugs, such as Wegovy, will be based on South Korea's health insurance finances, reimbursement equity, and the cost-effectiveness of these drugs.
- Opinion
- [Reporter’s View] Be aware, be prepared
- by Eo, Yun-Ho Sep 03, 2025 06:10am
- Even if it seems premature, there is nothing wrong with being prepared. Following the Trump administration’s executive order on the Most-Favored-Nation (MFN) drug pricing policy, the deadline for major multinational pharmaceutical CEOs to submit their proposed drug price reduction plans is fast approaching—September 29. The MFN drug pricing policy seeks to adjust U.S. drug prices to match the lowest price among advanced countries. Initially, this standard was set to apply to Medicaid—health insurance for low-income patients—and gradually expand to Medicare, the public health insurance program. In short, U.S. drug prices will be aligned with the lowest prices found among developed nations. Experts warn that South Korea could become that benchmark country. Given the already heightened concern about “Korea passing,” this may further push multinational companies to avoid listing their drugs in Korea’s reimbursement system altogether. The U.S. pharmaceutical market is the largest in the world and accounts for nearly half of the global market share, which is more than 20 times than that of Korea’s. For multinational firms pursuing profit, Korea becomes a market they can easily abandon. The signs are already visible. Since Trump’s drug pricing policy was announced, some multinational pharmaceutical companies have withdrawn evaluation applications for new drug listings, while others have temporarily halted headquarters’ approval for such applications. Even products already listed have been affected: in one case, a company withdrew authorization, leading to deletion from the reimbursement list. It is time to revisit solutions that have long remained at the “proposal” stage, such as list price preserving mechanisms and structural improvements in expenditure. For drugs already listed, policymakers need to explore alternatives to continuous post-listing price cuts imposed through current pricing mechanisms. Admittedly, dual pricing is an inherently self-serving policy that undermines transparency. By concealing actual country-to-country pricing, it widens the ambiguity of drug costs. Yet, it is also an unavoidable choice for a government seeking to protect its own citizens. Within this dilemma, Korea must now make its own rational choice. There is a chance the scope of Trump’s “bomb” will shrink compared to its initial form. But it remains a possibility nonetheless—even if positive signals were received at the Korea–U.S. summit. Being aware and being prepared is essential.
- Policy
- Luxturna shows signif improvement in 3 out of 6 patients
- by Lee, Tak-Sun Sep 03, 2025 06:09am
- Luxturna (voretigene neparvovec, Novartis), a one-shot gene therapy that costs approximately KRW 300 million, showed clinically significant changes set by reimbursement criteria in only half of the patients. As indicated in the performance evaluation results disclosed last year, the effectiveness was only 50%. The Health Insurance Review & Assessment Service (HIRA) disclosed the latest performance evaluation results for Luxturna on August 29. Luxturna is a gene therapy for patients with inherited retinal disease, administered as a single subretinal injection in each eye. The ceiling price for one vial is KRW 325.8 million, with a patient co-payment of approximately KRW 10.5 million per person. Due to its high cost, health authorities have a risk-sharing agreement (RSA) with three types of contracts (refund, expenditure cap, and performance-based refund) to manage the drug expenditure. The performance-based refund contract, in particular, requires a post-administration performance evaluation to adjust the refund rate. The detailed reimbursement criteria for this drug are as follows. 1. A clinical evaluation (light sensitivity, vision, visual field, etc.) must be performed before administration (within 90 days before the first eye's injection) and at 1-3 months, 12 months, and annually for up to 4 years after administration (after the second eye's injection if both eyes are treated). Objective records, such as medical charts, must be submitted. 2. Light sensitivity must be evaluated using a full-field light threshold test with white light. 3. A clinically significant change is defined as an improvement of 1 log unit (average value for both eyes) or more from the baseline in the full-field light threshold test. The first performance evaluation result was disclosed on October 31 of last year, following the reimbursement listing in February of that year. The first evaluation followed up on four patients 1-3 months after administration. The result showed that two patients had a significant improvement, while two did not, indicating a 50% success rate. The latest evaluation results are based on a total of six patient cases. The evaluation was conducted 1-3 months after administration for two patients and 12 months after for four patients. The results showed that one patient at 1-3 months and two patients at 12 months had a significant improvement. In contrast, it was evaluated that one patient at 1-3 months and two patients at 12 months did not meet the criteria for significant improvement. It means that half of the patients were successful, while the other half failed. Since Luxturna's performance evaluation will continue for up to 4 years, the efficacy is expected to be more accurately verified as more data accumulates. By adjusting the refund rate accordingly, the high cost of the drug can be controlled. HIRA is currently conducting performance evaluations for other high-cost drugs, including Kymriah, Zolgensma, and Qarziba.
- Policy
- Bill to mandate generic substitutions gains momentum
- by Lee, Jeong-Hwan Sep 03, 2025 06:09am
- On September 2, the ruling party introduced a bill to mandate generic (ingredient-based) prescriptions for medicines with unstable supply. The bill establishes the legal basis for “shortage drugs” and allows prescribing by ingredient name instead of brand name. The bill holds significance in that it amends the Pharmaceutical Affairs Act to define drugs frequently in shortage and sets out legal procedures to designate such through public-private consultations, and amends the Medical Service Act to legislate the legitimacy and standards for mandatory ingredient prescriptions. In other words, the bill enforces a law that stipulates that “drugs in such unstable supply that generic prescribing must be enforced” through deliberations by a public-private consultative body. Representative Jong-tae Jang of the Democratic Party of Korea, who spearheaded the amendments, has stated he will push for their swift passage. The participation of fellow Democratic Party representative Yoon Kim (a physician) and Rebuilding Korea Party Rep Sun-min Kim (also a physician) is expected to reduce obstacles in upcoming committee reviews. Specifically, the bill requires the Ministry of Health and Welfare (MOHW) to establish a Supply Management Committee for Shortage Drugs. After deliberation and resolution by this committee, the Minister of Health and Welfare will designate shortage drugs. The committee will have up to 30 members, including the Vice Minister of Health and Welfare as Chair, Deputy Commissioner of the Ministry of Food and Drug Safety (MFDS) as Vice Chair. The remaining 28 will consist of : ▲Senior officials from relevant central government agencies (appointed by Presidential Decree), ▲ representatives recommended by the Chair of the Korean Pharmaceutical Association, ▲ representatives recommended by the Korean Medical Association under Article 28 of the Medical Service Act, ▲ representatives recommended by the Korea Pharmaceutical and Bio-Pharma Manufacturers Association and other relevant industry groups, as well as ▲ experts with sufficient knowledge and experience. Thus, government officials, pharmacists, physicians, manufacturers, and academia will collectively decide which drugs require mandatory generic prescribing due to unstable supply. The Minister of Health and Welfare will also have dedicated staff and budget authority to designate and de-designate shortage drugs, monitor supply conditions, and implement distribution improvement measures. The bill also legalizes distribution interventions alongside generic prescribing. If supply is deemed significantly disrupted, or upon request from another central administrative agency head, the Minister may—after committee review—order measures to improve distribution regarding sales outlets, procedures, volumes, and conditions. Pharmacies, medical institutions, wholesalers, and other designated entities will be legally obligated to comply with these measures. However, the Minister must first consult with the Minister of Strategy and Finance and the Chair of the Fair Trade Commission before issuing such orders. The MOHW Minister will also build and operate a shortage drug management system. This will allow requesting and collecting information necessary for distribution control, which includes production, shipments, sales, prescriptions, and dispensing data, from manufacturers, importers, wholesalers, pharmacies, and medical institutions. This effectively grants the MOHW authority over the entire supply chain, but also increases the Ministry’s accountability when shortages occur. The Minister may also designate certain shortage drugs as “emergency production/import drugs”, subject to committee review, which empowers the Minister to order manufacturers to produce or import the drugs. The Medical Service Act amendment stipulates that physicians and dentists must prescribe designated shortage drugs by generic name, not brand name. Violation will result in up to 5 years imprisonment or a fine up to KRW 50 million. This significant level of criminal penalty is expected to draw strong opposition from the Korean Medical Association and the broader medical community.
- Company
- Moderna’s latest variant-targeted COVID-19 vaccine approved
- by Whang, byung-woo Sep 03, 2025 06:08am
- Moderna Korea announced on the 1st that its LP.8.1 variant-targeted COVID-19 vaccine, ‘Spikevax LP Inj’, has been approved by the Ministry of Food and Drug Safety (MFDS). Spikevax LP has been confirmed to induce broad cross-immune responses against currently circulating variants, including the LP.8.1 strain, and it is authorized for use in adolescents aged 12 and above as well as adults. Moderna plans to supply the newly approved vaccine in time for the government’s 2025–2026 seasonal immunization program, which begins in October. The company noted that its COVID-19 vaccines have demonstrated strong immune effect and safety in large-scale Phase III clinical trials and extensive real-world evidence (RWE). A key feature is that elderly individuals aged 65 and above showed immune responses comparable to those in younger adults. Also, regardless of which vaccine type had been administered previously, the Moderna vaccine showed high immunogenicity when used as a subsequent dose. A domestic study conducted by the Korea Disease Control and Prevention Agency (KDCA) also confirmed that Moderna’s vaccine recorded the lowest breakthrough infection rate among the vaccines used in the early stages of the pandemic. According to the National Immunization Program guidelines, Spikevax LP will be provided free of charge to high-risk groups, including seniors aged 65 and over and residents of long-term care facilities. The Korean Society of Infectious Diseases has stressed that immunity acquired through infection or vaccination wanes over time, and with the emergence of new variants, periodic updated COVID-19 vaccination for high-risk groups is recommended. The LP.8.1 series vaccines supplied this season have already been recommended for use by the World Health Organization (WHO), the European Medicines Agency (EMA), and the U.S. Food and Drug Administration (FDA). Based on recommendations from its Vaccination Expert Committee, the KDCA decided to adopt the LP.8.1 vaccine, which demonstrated stronger neutralizing antibody responses compared to last season’s JN.1-based vaccines. Moderna is the only company manufacturing mRNA COVID-19 vaccines in Korea, through its partnership with Samsung Biologics, and continues to ensure a stable vaccine supply via ongoing cooperation with Boryung Biopharma. Sang Pyo Kim, General Manager of Moderna Korea, said, “COVID-19 remains a threat to high-risk groups, with hospitalizations on the rise for 7 consecutive weeks. Moderna is committed to delivering updated COVID-19 vaccines that address the latest variants in a timely manner, ensuring that people can be vaccinated safely.”
- Company
- 'Prevenar 20' now available at general hospitals
- by Eo, Yun-Ho Sep 02, 2025 06:11am
- Product photo of Prevenar 20 PFS The pneumococcal conjugate vaccine 'Prevenar 20,' which will soon be included in the National Immunization Program (NIP), is becoming available for prescription at general hospitals. According to industry sources, Pfizer Korea's Prevenar 20 has passed the drug committees (DC) of tertiary general hospitals, including Samsung Medical Center, Seoul National University Hospital, Asan Medical Center in Seoul, and Sinchon Severance Hospital, and medical institutes, including Kangnam Sacred Heart Hospital, Kyung Hee University Hospital at Gangdong, Pusan National University Hospital, Seoul National University Bundang Hospital, Ajou University Hospital, Pusan National University Yangsan Hospital, and Chungnam National University Hospital. Several of these hospitals, including Seoul National University Hospital, have approved codes for adult patients only. However, as Prevenar 20 will be included in the NIP effective October, more hospitals are expected to have pediatric vaccines. Prevenar 20 is a pneumococcal conjugate vaccine that was approved by the Ministry of Food and Drug Safety (MFDS) on October 31, 2024. Compared to the 13-valent vaccine, Prevenar 20 has added seven additional pneumococcal serotypes. Among domestically approved pneumococcal conjugate vaccines, it contains the most serotypes. In addition to existing serotype components in the 13-valent vaccine, Prevenar 20 contains seven additional serotypes (serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F). It can be used to prevent invasive diseases and pneumonia in all ages, including infants aged six weeks and above. Pneumococcus is a major bacterial pathogen that causes various diseases in infants and young children, including otitis media, pneumonia, and meningitis. Vaccination is crucial, especially as it can cause life-threatening invasive pneumococcal disease (IPD) in immunocompromised children. Despite South Korea's NIP supporting the 23-valent pneumococcal polysaccharide vaccine (PPSV23) for adults aged 65 and older, this age group has the highest incidence of invasive pneumococcal disease (IPD). From September 2014 to mid-November 2023, a total of 3,734 cases of IPD were reported. The incidence rate for adults aged 65 and over was 32.1 cases per 100,000 people, accounting for 54.8% of all cases. This rate is the highest among all age groups under 65 years old. The inclusion of Prevenar 20 in the NIP was decided after a comprehensive review by the Korea Expert Committee on Immunization Practices (KECIP) on the vaccine's safety, immunogenicity, and cost-effectiveness. Meanwhile, Korea Vaccine is responsible for the domestic promotion of pediatric Prevenar 20, while Chong Kun Dang handles the promotion of the adult vaccine.
- Policy
- Kanarb and Faslodex to keep their price until ruling
- by Lee, Tak-Sun Sep 02, 2025 06:11am
- The drug prices for Boryung's hypertension treatment ‘Kanarb’ and AstraZeneca's anticancer drug ‘Faslodex,’ which were determined to be reduced by the MOHW due to the entry of generics, will be maintained at their previous levels for now. The court has decided to suspend the execution of the price reduction until the first-instance ruling. As a result, the industry’s eyes are on whether these pharmaceutical companies will be able to halt the price reduction disposition through the main lawsuit. According to industry sources on the 1st, the Seoul Administrative Court accepted an application on the 27th of last month to suspend the execution of the price reduction order for 11 items, including Kanarb Tab. Consequently, the previous insurance price ceiling for the drugs will be maintained until two months after the date of the final judgment of the main trial. The affected items are Boryung's Kanarb Tab (3 dosages), Kanarb Plus Tab (2 dosages), Dukarb Tab (4 dosages), and Dongwha Pharmaceutical's LaCor Tab (2 dosages). At the end of June, the MOHW announced an ex officio adjustment and termination of the premium for these items following the entry of generic drugs containing the active ingredient, the single-ingredient fimasartan. Implementation was scheduled for July 1st. Four pharmaceutical companies' Kanarb generic products were listed for reimbursement last May. Consequently, health authorities proceeded with the ex officio price reduction process for Kanarb. Despite Boryung's objection, the price adjustment was ultimately finalized. The price caps for Kanarb Plus Tab and LaCor Tab, which are combination drugs that contain fimasartan, were also administratively reduced due to the price adjustment. For Dukarb, the premium granted for incrementally modified new drug combinations ended as two or more fimasartan-based single-ingredient drugs became available. Kanarb’s price was reduced by 30%, Dukarb by 21%, and Kanarb Plus and LaCor by 47%. Given that Kanarb and Dukarb each generate sales in the KRW 60 billion range, the price adjustment is expected to lead to a significant decrease in Boryung's overall sales performance. Consequently, many believed Boryung would seek to provisionally maintain its drug prices through litigation. The first trial for the main suit will commence in earnest with the first hearing scheduled for November 13. Kim & Chang is representing Boryung. The drug price for AstraZeneca's anticancer drug Faslodex (fulvestrant) will also be maintained until 30 days after the ruling date of the main case. With more than three companies producing generic versions, Faslodex's price adjustment period was scheduled to end last July. As a result, its insurance price cap was set to decrease from KRW 376,724 to KRW 288,194 starting August 1. AstraZeneca was reportedly considering withdrawing from the domestic market due to the Faslodex price cut disposition. This issue also surfaced during the confirmation hearing for Minister of MOHW Eun-Kyung Jeong. Domestic pharmaceutical companies have also expressed the view that if the original anticancer drug withdraws, it would be difficult for patients to find a substitute, given the market characteristics. Fortunately, the company did not withdraw its product but opted for litigation. On the 29th of last month, the Seoul Administrative Court granted its application for a stay of execution of the price reduction order. The main lawsuit was filed on July 25th, and no hearing date has been set yet. AstraZeneca's legal representative is the law firm Sejong. Although both pharmaceutical companies chose litigation to maintain drug prices, the risk is not entirely absent. This is because the Act on Drug Price Litigation Recovery and Refund that was implemented last year allows the National Health Insurance Service to refund the claimed amount for the trial period if the company loses the case. Nevertheless, analysis suggests the companies ultimately chose litigation due to the significant immediate financial loss and the potential for dispute over the price reduction decision.
- Company
- Patent suits active to release Rinvoq generics
- by Kim, Jin-Gu Sep 02, 2025 06:10am
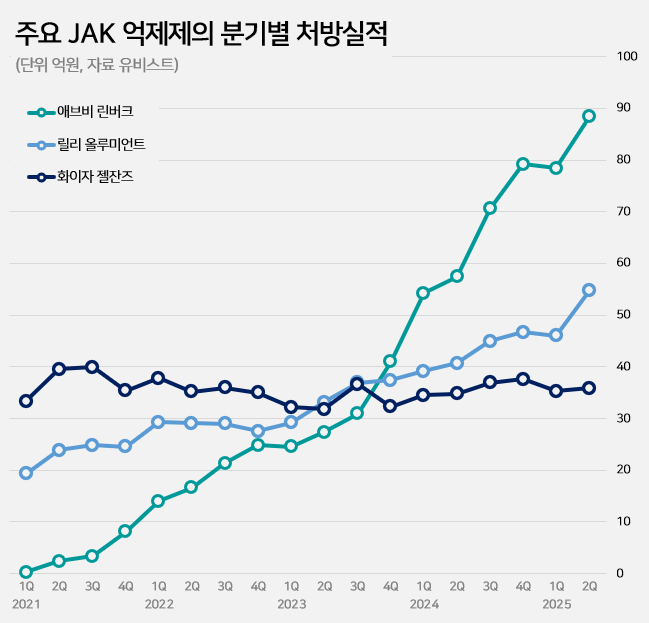
- Pic of Rinvoq The race to launch generics of AbbVie’s Rinvoq (upadacitinib), an oral treatment for autoimmune diseases, is showing signs of intensifying patent disputes. With the JAK inhibitor market expanding rapidly and Rinvoq solidifying its dominance, multiple companies are expected to mount patent challenges. According to industry sources on the 1st, Daewoong Pharmaceutical recently filed a passive scope confirmation trial against AbbVie, challenging the crystalline form patent of Rinvoq. Currently, two patents related to Rinvoq are registered: a substance patent expiring in May 2032 and a crystalline form patent expiring in October 2036. Daewoong's strategy is to first circumvent the crystalline form patent and then launch a generic version early, timed for the expiration of the substance patent. Chong Kun Dang opened the door to this dispute by challenging Rinvoq’s patents before Daewoong Pharmaceutical. Chong Kun Dang filed a related trial request on the 19th of last month. The pharmaceutical industry expects more companies to challenge Rinvoq’s patents from here on. One of the key requirements for obtaining first generic exclusivity is filing the initial invalidation trial. However, if another company files the same trial within 14 business days of the first filing date, it is also deemed to meet this requirement. This is why additional trial filings are expected in the coming weeks as companies move to secure first generic exclusivity for Rinvoq. According to UBIST, a pharmaceutical market research institution, outpatient prescriptions of JAK inhibitors in the first half of this year reached KRW 38.6 billion, up 40% from the KRW 27.5 billion in the same period last year. JAK inhibitors are experiencing rapid growth in the autoimmune disease treatment market, driven by the convenience of being oral formulations. The JAK inhibitor market, which was KRW 18.7 billion in 2020, grew by 36% to KRW 25.5 billion the following year. It continued to grow significantly each year: KRW 35.5 billion in 2022, KRW 40 billion in 2023, and KRW 62.2 billion in 2024. The expanded reimbursement granted for key products drove this market growth. Growth accelerated further last October when switching between JAK inhibitors for rheumatoid arthritis was allowed reimbursement. Major JAKi Prescriptions (AbbVie During this market expansion, Rinvoq has tightened its grip as the dominant player. In the first half of this year, Rinvoq recorded KRW 16.7 billion in prescriptions, a 49% increase from KRW 11.2 billion in the same period last year. Its growth has outpaced competitors such as Xeljanz (tofacitinib) and Olumiant (baricitinib). Having claimed the number one position in Q4 2023, Rinvoq has since expanded its market share, which now stands at 43% in the first half of this year. Rinvoq is a JAK inhibitor used for autoimmune diseases such as rheumatoid arthritis and atopic dermatitis. Its mechanism involves inhibiting inflammatory cytokines, thereby suppressing inflammation, pain, and cell activation.
- Company
- China's presence rises amid early-stage ADC candidate deals
- by Son, Hyung Min Sep 02, 2025 06:10am
- With competition in the antibody-drug conjugate (ADC) market intensifying, global deal trends are undergoing a clear shift. Transactions are now more active in the preclinical and early clinical stages, rather than in late-stage candidates, with collaborations between global pharma and Chinese companies standing out. Since the success of Enhertu, topoisomerase I (Topo I) payloads have effectively become the dominant approach. On the 29th, AimedBio and Samsung Medical Center held the 3rd ADC Conference at the hospital’s main auditorium, where AimedBio Chairman Nam-Gu Her highlighted recent changes in the ADC industry landscape. ADC therapies are novel anticancer drugs that link an antibody that is designed to bind a specific antigen on the surface of cancer cells with a cytotoxic payload through a linker. This design allows the drug to selectively target cancer cells, enhancing efficacy while minimizing adverse effects. According to Her, the ADC market has seen explosive growth since the launch of Enhertu in 2019. Over the past 5 years, deals worth USD 390 billion (KRW 540 trillion) have been completed, with more than 300 clinical trials initiated. The ADC Deal Boom and Trials Enhertu is a next-generation ADC composed of a monoclonal antibody that has the same structure as trastuzumab, which binds to receptors overexpressed on cancer cells and a highly potent Topo I inhibitor payload, linked via a tumor-selective cleavable linker. In a head-to-head study, Enhertu nearly doubled progression-free survival (PFS) compared to Kadcyla, which uses the same trastuzumab backbone but with a microtubule inhibitor payload. “Most companies are now developing ADCs with Topo I inhibitor payloads. In addition to Enhertu, Datroway, and Trodelvy are such examples,” said Her. In the past 5 years alone, 59 deals have been struck involving Topo I payloads, far outpacing tubulin inhibitors (28), degraders (11), immunomodulators (8), and DNA-damaging agents (5). Heo also noted that licensing dynamics have shifted. Historically, big deals centered on acquiring late-stage or commercial products. Today, however, activity has increased around preclinical candidates. High-profile examples made in the past include AstraZeneca’s partnerships on Enhertu and Datroway, AbbVie’s acquisition of Elahere, and Pfizer’s acquisition of Adcetris. However, nowadays, the same big pharma are actively scouting promising preclinical ADC assets. In the ADC payload development landscape, the use of topoisomerase I inhibitors has surged explosively. “The traditional deal trend was to acquire products rather than platforms. While deal sizes have decreased recently, interest in ADCs has not waned,” Her explained. “With many leading candidates already acquired or commercialized, transactions are now focusing on early-stage pipelines.” Chinese pharmaceutical companies are playing an increasingly prominent role. Technology exports from China surged from 55 cases in 2015 to over 300 in 2021, and recorded 213 last year. For instance, in 2022, MSD secured global rights to the TROP2 ADC sacituzumab tirumotecan from Kelun-Biotech for USD 1.41 billion. Last year, GSK obtained global rights to a B7-H3-targeting ADC from Hansoh Pharma. “The mainstream deal trend nowadays is acquiring on preclinical candidates through small-scale transactions. Global big pharma’s interest in Chinese firms is growing rapidly, reflecting China’s strengthened competitiveness in the ADC development scene.”