- LOGIN
- MemberShip
- 2025-12-20 04:35:51
- Company
- Eylea high-dosage PFS formulation wins nod in Korea
- by Whang, byung-woo Aug 29, 2025 06:04am
- Bayer Korea announced on August 28 that it has received domestic approval for 'Eylea PFS 8mg (aflibercept),' a pre-filled syringe formulation, from the Ministry of Food and Drug Safety (MFDS). The indication for 'Eylea PFS 8mg' has been approved the same as that for the existing Eylea vial formulation. Now, it can be administered more simply and accurately. This drug is indicated for the treatment of visual impairment due to neovascular (wet) age-related macular degeneration (nAMD) and diabetic macular edema (DME). Additionally, the Eylea PFS 8mg comes with Bayer's PFS device, OcuCLICK. OcuCLICK is designed to mechanically inject the recommended dose of 0.07ml precisely into the vitreous cavity, helping to shorten injection preparation time for healthcare professionals and minimize dosing errors. This mechanism enhances the accuracy and convenience of the procedure, providing a safe administration environment for both patients and healthcare providers. Eylea 8mg has a molar dose four times higher than the previous version, allowing it to maintain an effective concentration in the eye for an extended period. After monthly injections for the initial three months, the injection interval can be extended up to 20 weeks, depending on the patient's condition. The latest PFS formulation is expected to reduce the number of injections for patients, thereby easing their treatment burden, while also improving the efficiency of procedures for healthcare professionals. Hyun-Mi Han, Lead of Ophthalmology Therapy Portfolio at Bayer Korea, stated, "Eylea PFS 8mg is a significant advancement that not only improves patients' treatment experience but also brings about a groundbreaking shift in the medical paradigm," and added, "As a leader in the ophthalmology field, Bayer will continue to provide innovative solutions that reduce the burden on patients and healthcare professionals, in response to the growing demand from patients with nAMD and DME due to the super-aging population." Meanwhile, Eylea PFS 8mg has been approved and launched in major markets, including the EU, Japan, and Canada, and is being utilized in real-world clinical practice.
- Company
- "200-day extension for the CMV preemptive therapy Prevymis"
- by Whang, byung-woo Aug 28, 2025 06:10am
- Prevymis (letermovir), a treatment for preventing cytomegalovirus (CMV) infection in allogeneic hematopoietic stem cell transplant patients, is bringing in another paradigm shift as it has entered the '200-day extension criteria.' This drug's influence is expected to grow as the National Health Insurance (NHI) coverage period has been extended from the previous 100 days post-transplant to 200 days. DailyPharm met with Professor Dong-Gun Lee of Seoul St. Mary's Hospital's Division of Infectious Diseases (President of the Korean Society of Infectious Diseases) to discuss the significance of Prevymis's reimbursement and future challenges. The launch of Prevymis has brought a paradigm shift in infection management Professor Dong-Gun Lee of Seoul St. MaryCMV is a type of herpesvirus that lies dormant in the human body. Although it's common enough that 95% of Korean adults are seropositive for its antibodies, it is one of the most fatal infections for patients with weakened immunity due to hematopoietic stem cell transplantation or other factors. Notably, CMV is reactivated in about two-thirds of patients within the first three to four months post-transplant. This can lead to serious complications such as pneumonia, gastroenteritis, retinitis, myelitis, and myelosuppression, and in some cases, can result in death. Prevymis was approved by the Ministry of Food and Drug Safety (MFDS) in 2018 and approved for reimbursement in Korea in September of the same year. It is regarded as leading a paradigm shift from a preemptive therapy, defined as administering antiviral drugs to prevent infection, to a preventive therapy using Prevymis. Professor Lee explained, "Typically, in 5 out of 10 allogeneic hematopoietic stem cell transplant patients, CMV is reactivated, and they endure lengthy anti-cancer treatments of up to 9 months and multiple hospitalizations before transplantation." He added, "Among existing antivirals, ganciclovir is an intravenous injection, requiring re-hospitalization for administration. Even with the oral valganciclovir, severe side effects like leukopenia often made it difficult to avoid hospitalization." In this context, Professor Lee explained, Prevymis, which is available in both injectable and oral formulations and has a low toxicity burden, made it possible to continue preventive treatment without hospitalizing the patient. It is particularly praised for its significant role in improving patient survival rates and treatment success rates by reducing the CMV reactivation rate to less than one-third of what it was, thereby significantly delaying the occurrence of CMV disease and the associated risk of death. According to Professor Lee, when Prevymis was administered preventively, the reactivation rate within 100 days was lowered to about 5-6% in a U.S. clinical trial, and it was confirmed to be around 12-13% in data from Seoul St. Mary's Hospital. Professor Lee stated, "If acute graft-versus-host disease (GVHD) occurs within approximately 100 days post-transplant, the patient's immune function is compromised, not only leading to CMV reactivation but also increasing the risk of other infections." He added, "If CMV is prevented during this period with Prevymis, the occurrence of CMV infection and related complications can be reduced." Extended Prevymis Administration Shows Clear Efficacy, Prevents CMV Reactivation There were also limitations to the 100-day prevention regimen with Prevymis. While the CMV reactivation rate decreased from the previous 50% to approximately 11-12% when Prevymis was administered for up to 100 days, the reactivation rate rose again once the drug was discontinued. Data from Seoul St. Mary's Hospital showed that approximately one-third of patients experienced CMV reactivation between 100 and 200 days after completing the 100-day prevention regimen. This means that since immunosuppression persists for up to six months post-transplant, and the risk of other viral or fungal infections remains high, patients can be exposed to various infection risks if there is a gap in preventive medication. Professor Lee emphasized, "Extending the administration period to 200 days lowers the reactivation rate that occurs after 100 days to about 11%." He added, "This means that depending on how long Prevymis is administered, the timing of CMV reactivation can be delayed accordingly." Based on this, the criteria for Prevymis reimbursement were expanded from 100 days to 200 days, effective June 1. Reimbursement is limited to high-risk adult patients who are CMV seropositive after allogeneic hematopoietic stem cell transplantation. Professor Lee stated, "High-risk groups include patients with GVHD or those who received high-intensity conditioning regimens, and these are the patients eligible for the 200-day extended administration." He added, "In fact, more than 7-8 out of 10 hematopoietic stem cell transplant patients at Seoul St. Mary's Hospital fall into the high-risk group, and most of them receive extended Prevymis administration for up to 200 days." In a pivotal Phase 3 study, which served as the basis for Prevymis's expanded reimbursement, the patient group that continued the prevention regimen for up to 200 days showed a 16.1% lower incidence of CMV infection compared to the placebo group. It also demonstrated a safety profile comparable to that of the placebo group during long-term administration. Professor Lee emphasized, "During the recovery period, when immunity is compromised, there is a high risk of co-infections with other infectious diseases (respiratory viruses, other bacteria or viruses, and fungal infections) in addition to CMV. Extending Prevymis administration to 200 days to prevent CMV reactivation until the immune system recovers can also contribute to reducing the possibility of co-infections." Despite expanded reimbursement criteria, unmet needs remain However, challenges still exist. According to the reimbursement criteria, extended administration beyond 200 days is not possible for patients who are not classified as high-risk. In clinical practice, the high-risk status is meticulously evaluated around the 100-day mark to decide whether to extend the administration. The problem arises in cases where patients are not high-risk at 100 days and stop taking the drug, only to see their risk increase later due to GVHD or other factors. Professor Lee pointed out that under the current criteria, Prevymis cannot be re-administered to the same patient once it has been stopped. This results in an unmet need, as patients who require ongoing treatment are unable to receive it. He stated, "Although continued administration would be clinically beneficial, there are actual cases where we cannot use Prevymis due to concerns about reimbursement cuts," and added, "Ultimately, these patients are exposed to the risk of CMV reactivation when their immunity is suppressed." Overseas, there are reports of re-administering Prevymis within 200 days after a temporary treatment with other drugs. However, Professor Lee advised that this is not easy in Korea due to concerns about reimbursement cuts, necessitating additional institutional improvements. Finally, as President of the Korean Society of Infectious Diseases, Professor Lee emphasized the need for a change in the public perception of preemptive or preventive drug administration for infectious diseases, which is often viewed as drug misuse or abuse. Professor Lee explained, "For patient groups with severely compromised immunity, such as transplant or cancer patients, treating the disease after it has occurred is too late and dangerous," and added, "Preventive treatment for diseases with a high risk of onset is a matter of life and death for patients." Professor Lee added, "Perceiving this as drug misuse overlooks the characteristics of infectious diseases and the reality of high-risk patients. Given that a significant portion of antibiotic misuse is in non-medical areas like livestock farming, we need to adjust the perception that attributes the cause of antibiotic resistance solely to doctors' misuse."
- Company
- Kolon Pharma's active portfolio expansion…new drug·co-dev
- by Son, Hyung Min Aug 28, 2025 06:10am
- Kolon Pharma is increasing its efforts to secure competitiveness in immunology and oncology by combining new drug in-licensing with in-house R&D. Following the introduction of treatments for chronic obstructive pulmonary disease (COPD) and allergies, the company has recently secured a new drug for hypoparathyroidism. Kolon Pharma plans to continuously expand its portfolio through its own pipelines and co-development of new anti-cancer drugs. Active In-Licensing of Drugs for Immune Diseases According to industry sources, on August 27, Kolon Pharma recently secured the exclusive domestic sales rights for 'YORVIPATH,' a treatment for hypoparathyroidism, from Ascendis Pharma of Denmark. YORVIPATH is an innovative new drug that received approval in Europe in 2023, the U.S. in 2024, and Australia this year. Hypoparathyroidism is a rare disease in which a lack of parathyroid hormone (PTH) disrupts the balance of calcium and phosphorus in the blood. Until now, patients have relied on taking dozens of calcium and active vitamin D tablets a day, but this has been problematic due to the long-term strain on the kidneys and incomplete symptom control. YORVIPATH is characterized by a mechanism that addresses the fundamental cause of the disease by naturally secreting PTH over 24 hours. Following its FDA approval, over 3,000 patients are receiving treatment as of Q2 2025. Commercialization is also expanding in Europe and Australia. COPD dual comb therapyKolon Pharma's new drug-licensing strategy has already been proven effective in immunological diseases, such as COPD and allergies. The company in-licensed the COPD combination products Foster (dual comb therapy) and Trimbow (triple comb therapy) from Italy's Chiesi, securing domestic approval in 2019 and establishing them in the market after reimbursement. In 2020, Kolon Pharma introduced Bilastine from Spain's Faes Farma, creating a new dynamic in the domestic allergy treatment market. Bilastine is a new antihistamine drug that is currently sold in over 100 countries. Kolon Pharma has also in-licensed new drugs in the gastroenterology and dermatology sectors. In 2011, the company launched Clipper SR Tabs, a locally-acting oral steroid for ulcerative colitis, in Korea. In 2012, Kolon Pharma also introduced Veregen Ointment, an HPV (human papillomavirus) treatment based on green tea extract, strengthening its dermatology and urology drug lines. Veregen Ointment has established itself as a differentiated treatment option, as it has been proven to not only treat common warts but also reduce the recurrence rate. Kolon Pharma Continues Co-Development and In-house New Drug Development Efforts Kolon Pharma is not stopping at simply in-licensing in the immunological field. The company is also strengthening its own capabilities in new drug development. In 2023, Kolon Pharma signed a co-development agreement with GBiologics for GB930, a treatment for systemic lupus erythematosus. Kolon Pharma signed a co-development agreement with Gbiologics for the treatment of systemic lupus erythematosus GB930 is a new drug candidate based on a stabilized galectin-9 protein, which has a dual-action mechanism that simultaneously suppresses B cells and plasmacytoid dendritic cells. The two companies aim to submit an Investigational New Drug (IND) application to the U.S. FDA and are also open to global out-licensing opportunities. Kolon Pharma is also venturing into the development of new anti-cancer drugs. Last year, the company partnered with Estrium, a small molecule drug development company, to co-develop AON-MB23, a new drug for triple-negative breast cancer (TNBC). AON-MB23 is a new drug candidate that offers new possibilities for TNBC, for which treatment options are limited. Preclinical studies are underway to submit an IND in 2027. TNBC is an aggressive subtype that accounts for 15-20% of all breast cancers, making it an area of great interest in the global market due to high treatment demand. Additionally, Kolon Pharma partnered with Aptamer Sciences last year to co-develop the Antibody-Drug Conjugate (ADC) candidate 'AST-203.' The two companies plan to conduct clinical studies on AST-203 to secure a pancreatic cancer indication. AST-203 targets the TROP2 protein, which is primarily expressed in breast, pancreatic, gastric, and lung cancers. This new drug candidate has a mechanism that selectively binds to TROP2-positive tumors, penetrates the cell, and releases the cell-division-inhibiting drug MMAE, thereby inducing cancer cell death. In addition to co-development, Kolon Pharma is also securing its medium- to long-term growth engines through in-house new drug development. Active compound identification and optimization for PBS203, an in-house development project, was completed in 2021, and the project entered the CMC (Chemistry, Manufacturing, and Controls) and preclinical stages in 2022. After submitting an IND in 2023, it entered the clinical stage, with clinical trials having been underway since last year. Currently, PBS203 is in clinical trials for solid tumors, including pancreatic and colorectal cancer. Kolon Pharma is also developing PBL201 and PBL211 for major solid tumors, such as pancreatic cancer and melanoma.
- Policy
- Bill banning reverse payment agreements passes committee
- by Lee, Jeong-Hwan Aug 28, 2025 06:10am
- The amendment to the National Health Insurance Act, which regulates "reverse payment agreements" where original drug companies and generic drug companies collude by exchanging money to delay or not release generics, thereby avoiding a price reduction for the original drug, passed the National Assembly Health and Welfare Committee review on the morning of the 27th. The illegal collusion among pharmaceutical companies to withhold generic drug launches has forced patients to pay unfairly high prices for medications. This practice must be eradicated to eliminate such disadvantages and prevent unnecessary leakage of health insurance funds. The bill on revising the Pharmaceutical Affairs Act, which expands the post-notification system for generic substitution at pharmacies to the information system established and operated by the Ministry of Health and Welfare and the Health Insurance Review and Assessment Service, and improves the definition of essential medicines and expands support to include drugs with no substitutes or facing supply instability, has also cleared the Health and Welfare Committee. These bills will take effect according to the implementation date specified in the supplementary provisions once they pass the plenary session after review by the Legislation and Judiciary Committee and are promulgated by the government. Original-Generic Pharma Company Collusion Prohibition…Price cuts or reimbursement suspensions applied for offenses The core provision of the National Health Insurance Act amendment bill proposed by Democratic Party of Korea lawmaker Young-seok Seo stipulates that if the Fair Trade Commission uncovers collusion where a generic drug company agrees not to launch a generic in exchange for exclusive domestic distribution rights from an original drug company, the price of the unfairly traded drug will be reduced or its reimbursement suspended. The bill passed by the Health and Welfare Committee amends Article 41-2 (Reduction of Upper Limit Amount for Reimbursement Costs for Drugs, etc.) of the National Health Insurance Act. Specifically, the bill stipulates that for cases violating Article 40(1) or Article 45(1) of the Monopoly Regulation and Fair Trade Act, where the violation was committed “for the purpose of increasing or maintaining the reimbursement price ceiling for drugs,” the insurance price of the drug can be reduced or reimbursement suspended. The amendment allows for a maximum 20% reduction in drug prices when a reverse payment agreement violation is first detected. If another reverse payment agreement is confirmed within 5 years of the price reduction, the price can be cut by up to 40%. If a second violation of the reverse payment agreement is detected within five years after the second price reduction, the application for reimbursement for the drug can be suspended for up to one year. The effective date for the provisions preventing reverse payment agreements is ‘the day six months after the government's promulgation’. Consequently, collusive reverse payment agreements will now face the same level of penalties as illegal pharmaceutical rebates, including drug price cuts and reimbursement suspensions. The Health and Welfare Committee explained, “This bill enables the reduction of the maximum reimbursement amount for drugs related to unfair joint conduct or unfair trade practices, and the suspension of reimbursement coverage. It will prevent pharmaceutical companies and others from profiting through unfair joint conduct or unfair trade practices and establish a fair drug sales order.” Post generic substitution notification system expanded to HIRA A revision to the Pharmaceutical Affairs Act, proposed by Democratic Party of Korea lawmakers Young-seok Seo, Su-jin Lee, and Byung-duk Min, was passed as an alternative bill by the Health and Welfare Committee. It expands the pharmacists’ post-dispensing notification system to the information system operated by the Health Insurance Review and Assessment Service (HIRA). The bill incorporates the existing regulation requiring pharmacists to inform patients when substituting a drug listed on a prescription with an item recognized by the Ministry of Food and Drug Safety as bioequivalent (generic substitution) and to notify the prescribing physician or dentist within one day (or three days if unavoidable circumstances exist). The key provision approved by the Health and Welfare Committee is the establishment of a new Article 27-2 (Establishment and Operation of a Substitution Dispensing Information System) in the Pharmaceutical Affairs Act, enabling the Minister of Health and Welfare to establish and operate an information system to support post-notification of generic substitution.. Notably, the bill allows the Minister of Health and Welfare to delegate this task to the Health Insurance Review and Assessment Service (HIRA), with the necessary details to be stipulated under the Ministry of Health and Welfare ordinance. The effective date for the simplified generic substitution regulations is set for five months after the government's promulgation. This establishes the legal basis for the Ministry of Health and Welfare and HIRA to build and operate a generic substitution information system to support post-notification of generic substitution under the Pharmaceutical Affairs Act. It is expected that this will enable more efficient communication of generic substitutions while clarifying the accuracy of such notifications, thereby enhancing information sharing between physicians and pharmacists. Expansion of the Definition of Essential Medicines & Legal Basis for the National Essential Medicines Stable Supply Council The Committee also passed a revision to the Pharmaceutical Affairs Act that improves and expands the definition of essential medicines to include those without substitutes or facing supply instability, and elevates the legal basis for the National Essential Medicines Stable Supply Council from a presidential decree to a law. The bill provides a detailed definition of essential medicines. These include medicines essential for maintaining the national health system, such as disease control and radiation disaster prevention (Item a), and medicines that are essential for healthcare but lack substitutes or face supply instability despite market mechanisms (Item b). Furthermore, a new Article 83-5 (National Essential Medicines Stable Supply Council) was added to the Pharmaceutical Affairs Act. This mandates the establishment of a National Essential Medicines Stable Supply Council within the Ministry of Food and Drug Safety (MFDS) to develop countermeasures for both national essential medicines and medicines that, while not designated as such, require stable supply due to temporary increases in demand. Notably, the composition of the National Essential Medicines Supply Council, previously chaired solely by the MFDS Vice Minister, was expanded to include ‘one senior official from the Ministry of Health and Welfare designated by the Minister of Health and Welfare,’ bringing the total to two chairs. This appears aimed at strengthening national management capabilities by adding the Ministry of Health and Welfare to the MFDS as a government ministry with authority over national essential medicines. Furthermore, the legal basis for the composition of the council members was clarified. The effective date of the bill was set as one year after its promulgation. While the definition of essential medicines is being expanded, the government's basis for supporting medicines without substitutes and medicines with unstable supply requiring stable supply due to temporary increases in demand is expected to be strengthened.
- Opinion
- [Reporter’s View] Addressing the Drug Shortage Issue
- by Kim JiEun Aug 28, 2025 06:09am
- With the bill that revises the Pharmaceutical Affairs Act, centered on simplifying generic substitution notifications, recently passing the NA Health and Welfare Committee's Legislative Subcommittee review, the Korean Medical Association readily voiced its opposition. This amendment primarily expands the scope of post-substitution notifications from pharmacies to include the information system operated by the Health Insurance Review and Assessment Service (HIRA). If passed, it is expected to provide the legal basis for implementing the simplified generic substitution notification enforcement rules under the Pharmaceutical Affairs Act, scheduled to take effect on February 2 next year. As soon as the amendment cleared the National Assembly's subcommittee hurdle, the KMA readily protested, calling it a harmful law that facilitates generic substitutions, and immediately demanded its withdrawal. The KMA used the term ‘arbitrary generic substitutions,’ arguing that implementing a related system would disregard physicians' prescribing authority. This reaction from doctors was not unexpected. Medical associations, including the KMA, have consistently opposed not only international nonproprietary name prescribing but also any systemic improvements related to simplifying generic substitutions. The reasons doctors have consistently cited for opposing the promotion of generic substitutions include threats to patient safety from pharmacist-initiated prescription changes, the undermining of doctors' prescribing authority, and the consequent collapse of the separation of prescribing and dispensing. Setting aside the claim that it would undermine physicians' prescribing authority, the argument that promoting generic substitution threatens patient safety and undermines the foundation of the separation of medical and pharmaceutical practices is difficult to accept. The drug supply issue has persisted for over five years since the spread of COVID-19. Even if the severity of the issue has lessened somewhat compared to the COVID period, unpredictable drug shortages continue to occur simultaneously and persistently. As a result, not only the pharmaceutical and distribution industries but also pharmacies are devoting a significant portion of their operations to securing drug inventories and managing supply. Had it not been for the efforts of frontline pharmacists to secure drug inventories during the severe COVID-era shortages, along with attempts at generic substitutions and patient understanding, the patients’ ‘pharmacy runaround’ – which might have otherwise been a temporary issue – could very well have escalated into a major societal problem threatening patient safety. Drug shortages and out-of-stock issues have persisted for years without effective countermeasures, yet the government has failed to present clear alternatives, and relevant legislation remains indefinitely stalled in the National Assembly. In this process, this reporter must ask: What voices have doctors, who emphasize maintaining prescription authority, raised for patient safety? What alternatives have they proposed? Claiming rights inevitably entails corresponding duties and responsibilities. To secure the authority to prescribe medications, there must also be a duty and responsibility to contribute to creating an environment where prescribed drugs can be delivered to patients without incident. Asserting rights without responsibility can only be perceived as an abuse of authority. The government must now focus its full efforts on establishing the institutional foundation to ensure that generic substitutions—which it has sought to promote, even introducing incentive systems—can truly become ‘activated,’ without being swayed by the claims of specific professions.
- Company
- New ADC drug introduced…expands treatment options
- by Son, Hyung Min Aug 28, 2025 06:09am
- New global drugs are awaiting entry into Korea’s antibody-drug conjugate (ADC) market one after another. Following Daiichi Sankyo Korea's application for domestic approval of the ADC anticancer drug ‘Datroway,’ AbbVie Korea is also proceeding with the approval process for its ovarian cancer-targeted ADC ‘Elahere.’ The industry anticipates that the commercialization of these two new drugs will significantly accelerate the competitive landscape for ADCs in Korea. According to industry sources on the 25th, Daiichi Sankyo Korea has completed its application for the domestic approval of its ADC anticancer drug Datroway and is awaiting approval. The indication is for hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. The company expects approval early next year. ADC anticancer drug Datroway Dartroway is an ADC being co-developed by Daiichi Sankyo and AstraZeneca. AstraZeneca has prior experience commercializing the HER2-targeted ADC ‘Enhertu’ with Daiichi Sankyo. Previously, AstraZeneca secured the development and sales rights for Enhertu through a total USD 6.9 billion (KRW 8.418 trillion) contract with Daiichi Sankyo. In 2020, AstraZeneca paid Daiichi Sankyo USD 1 billion (KRW 1.22 trillion) as an upfront payment to obtain the development rights for Datroway. The total contract value, including development milestones and commercialization milestones, amounts to USD 6 billion (KRW 7.32 trillion). TROP2, which Datroway targets, is rapidly emerging as a global ADC target. The TROP2 protein is overexpressed in various cancers, including breast cancer and non-small cell lung cancer. Datroway binds to this protein and delivers a cytotoxic drug into cancer cells, inducing cell death. It maintains the efficacy of existing cytotoxic anticancer drugs while reducing damage to normal cells. The first novel drug to reach commercialization with this mechanism was Gilead's Trodelvy. Trodelvy has been approved as a TROP2-targeted breast cancer treatment in the US, Europe, and South Korea. Subsequently, Datroway entered the market, adding indications for breast cancer and non-small cell lung cancer. Datroway demonstrated efficacy in the Phase III TROPION-BREAST01 clinical trial. This study was a randomized 1:1 trial comparing the Datroway group with the investigator-selected chemotherapy group (eribulin, vinorelbine, capecitabine, or gemcitabine) in patients with previously treated, unresectable or metastatic hormone receptor-positive (HR+/HER2-) breast cancer. The study included 723 patients with a median age of 56 years. Key endpoints included progression-free survival (PFS), defined as the time without disease progression as assessed by blinded independent central review (BICR) per RECIST 1.1, and overall survival (OS), defined as the time from treatment initiation to death. Results showed the median PFS in the Datroway group was 6.9 months. This was longer than the 4.9 months observed in the chemotherapy group. Although the OS data were immature, a trend favoring Datoray was observed. AbbVie's first ADC ‘Elahere nears domestic commercialization ADC anticancer drug Elahere AbbVie is also awaiting approval for a new ADC. AbbVie has applied for domestic approval of ‘Elahere’ targeting the ovarian cancer indication. AbbVie acquired Elahere in November 2023 by purchasing the US biotechnology company Immunogen for USD 10.1 billion (approximately KRW 13.1 trillion). Elahere is an ADC targeting ovarian cancers expressing FRα (folate receptor alpha). Its mechanism involves delivering the potent cytotoxic drug DM4 into cancer cells to destroy the tumor. It is particularly gaining attention as a new option for ovarian cancer patients resistant to platinum-based anticancer drugs. This treatment was also designated as an orphan drug in Korea this January. For epithelial ovarian cancer, which accounts for 90% of ovarian cancers, taxane-based drugs like paclitaxel and platinum-based anticancer drugs like carboplatin and cisplatin are primarily used. However, for recurrent ovarian cancer resistant to platinum-based drugs, response rates to standard chemotherapy have generally been low, significantly limiting improvements in survival rates. Elahere demonstrated its potential as a new alternative through the Phase III MIRASOL study, conducted in patients with platinum-resistant ovarian cancer. The confirmatory Phase III MIRASOL study enrolled 453 patients with platinum-resistant epithelial ovarian cancer. The trial compared the Elahere group with the standard chemotherapy group. At a median follow-up of 30.5 months, Elahere demonstrated an improvement in median PFS of 5.59 months compared to 3.98 months in the standard therapy group. This represented a 37% reduction in the risk of disease progression or death. The objective response rate (ORR), which measures the proportion of patients with tumor size reduction over a set period, was also higher in the Elahere group at 41.9%, compared to 15.9% in the standard therapy group. Overall survival (OS) was also longer with Elahere at 16.85 months, reducing the risk of death by 32% compared to 13.34 months in the standard therapy group. Regarding safety, eye-related adverse events, fatigue, and abdominal pain were reported, but these were generally considered manageable. Based on these results, Elahere received full approval in the United States in March of last year and obtained marketing authorization in Europe in November of the same year.
- Policy
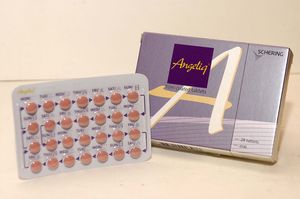
- Reimb listing approved for the first generic of 'Angeliq'
- by Lee, Tak-Sun Aug 27, 2025 06:07am
- Bayer The first generic of Angeliq Tab (drospirenone·estradiol, Bayer), a hormone-based medicine for use in postmenopausal women, will be included in the reimbursement listing. Analysis suggests that the commercialization of domestically produced generic is significant, considering that there had been supply issues related to Angeliq, which is an imported medicine. According to industry sources on August 26, Dalim BioTech's 'Anzeno Tab' will be added to the reimbursement list on September 1 with a ceiling price of KRW 5,565. The product is manufactured directly by Dalim BioTech. Anzeno Tab is a generic drug with the same active ingredients as Bayer's Angeliq Tab. Angeliq is approved for ▲Hormone replacement therapy for estrogen deficiency in women who are at least one year post-menopause, and for the ▲Prevention of osteoporosis in post-menopausal women who are intolerant of or have contraindications to other approved drugs and have an increased risk of fracture. Based on 2024 UBIST data, Angeliq's outpatient prescription sales amounted to KRW 12 billion. Because it is a hormonal drug requiring a separate manufacturing facility had previously prevented generic products from entering the market. Consequently, when supply issues arose with the imported original drug, Angeliq, pharmacies had no identical alternatives, leading to significant difficulties. Alternative prescriptions with similar drugs were the only option, resulting in a continuous demand for generic development. In 2021, the persistent shortage of Angeliq due to production delays at Bayer's Berlin plant caused difficulties for pharmacists. With the launch of this in-house-manufactured generic, the issue of supply instability is expected to be largely resolved. The price of Anzeno Tab was set at KRW 5,565, which is 53.55% of Angeliq's price, without any additional premium, as it met all the required criteria. Angeliq Tab's current ceiling price is KRW 10,393.
- Company
- Jaypirca’s reimbursement imminent in Korea
- by Eo, Yun-Ho Aug 27, 2025 06:07am
- The BTK inhibitor Jaypirca is likely to be listed for reimbursement soon. According to Dailypharm coverage, the National Health Insurance Service (NHIS) and Lilly Korea recently completed price negotiations for Jaypirca (pirtobrutinib), a treatment for relapsed or refractory mantle cell lymphoma (MCL). As a result, Jaypirca’s reimbursement agenda is scheduled to be submitted to the Health Insurance Policy Deliberation Committee in September, with listing expected in October. Jaypirca was approved by the Ministry of Food and Drug Safety in August last year as a monotherapy for adult patients with relapsed or refractory mantle cell lymphoma (MCL) who have previously received two or more treatments, including a BTK inhibitor. Prior to its approval, there were no approved medications available for patients with relapsed or refractory MCL whose disease progressed after treatment with existing BTK inhibitors in Korea. Jaypirca is the first and only reversible BTK inhibitor that has demonstrated clinical efficacy in patients with relapsed or refractory MCL following treatment with one or more BTK inhibitors. It also exhibits a 300-fold higher selectivity for BTK compared to most kinases (98%) included in preclinical studies. The BRUIN Phase I/II clinical trial, the study that became the grounds for Jaypirca’s approval, evaluated the clinical efficacy and safety of Jaypirca in adult patients with relapsed or refractory mantle cell lymphoma who had previously received treatment with one or more BTK inhibitors. In the primary analysis set (PAS) of 90 patients who had previously received treatment with one or more BTK inhibitors, the overall response rate (ORR) was 56.7%, with a median duration of response (DoR) of 17.6 months. The most common adverse reactions following Jaypirca administration were fatigue (26.3%), neutropenia (22.8%), diarrhea (22.1%), and bruising (19.0%). The incidence of treatment discontinuation due to adverse reactions was 1.2%, and the incidence of dose reduction due to adverse reactions was 3.3%. Meanwhile, based on the response rate results, Jaypirca was approved through the U.S. FDA's accelerated approval process in January last year. In Korea, it was designated as an orphan drug in June last year for use as monotherapy in adult patients with relapsed or refractory MCL who had previously received treatment with a BTK inhibitor.
- Company
- Targeted therapies and antibody drugs enter clinical trials
- by Son, Hyung Min Aug 27, 2025 06:07am
- Korea’s pharmaceutical and biotech industry is accelerating new drug development for pancreatic cancer, a cancer regarded as one of the most intractable cancers. Onconic Therapeutics, Prestige Biopharma, and Aptamer Sciences have each started clinical trials, making their bid into the field. The company plans to test the commercial viability of various mechanisms in the area, including antibody-drug conjugates (ADCs), targeted anticancer agents, and antibody-based novel therapies. Pancreatic cancer is difficult to detect at an early stage and has a poor prognosis, with a five-year survival rate of only 12.6 percent — the lowest among the nation’s top ten cancers. Given the limited success seen with existing therapies, there is a growing consensus that the development of new drugs is urgently needed. Onconic Therapeutics applies to initate Phase II trial in Korea According to industry sources on the 26th, Onconic Therapeutics has recently submitted an Investigational New Drug (IND) application to the Ministry of Food and Drug Safety for a Phase II clinical trial of its anticancer drug candidate Nesuparib. Nesuparib is a novel drug candidate with a dual mechanism of action, simultaneously inhibiting poly (ADP-ribose) polymerases (PARPs) and tankyrases (TNKS). The compound is currently being evaluated as both a monotherapy and as combination therapy across multiple indications, including pancreatic, endometrial, and gastric cancers. It has also been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Administration (FDA) for pancreatic and gastric cancers. Onconic Therapeutics has confirmed the maximum tolerated dose (MTD) and the recommended Phase II dose (RP2D) for its anticancer candidate in a Phase Ib clinical trial involving patients with advanced or metastatic pancreatic cancer. This multicenter, open-label, Phase Ib dose-escalation study enrolled up to 48 patients with locally advanced or metastatic pancreatic cancer. Participants were divided into two cohorts - Arm A, which received Nesuparib in combination with the FOLFOXIRI regimen (oxaliplatin, leucovorin, irinotecan, and 5-fluorouracil (5-FU)), and Arm B, which received Nesuparib in combination with gemcitabine and nab-paclitaxel. Onconic Therapeutics described the results of the Phase Ib trial as encouraging and announced its intention to present the findings at upcoming major international oncology conferences. New antibody drug development active in the industry… companies also see potential in combination therapies Korea’s pharmaceutical and biotech industry is stepping up its efforts in the field, with companies pursuing a wide range of new drug candidates, including antibody-drug conjugates (ADCs) and novel antibody therapies. Aptamer Sciences recently unveiled preclinical data for its ADC candidate AST-203, which targets TROP2, a protein commonly expressed in breast, pancreatic, gastric, and lung cancers. AST-203 selectively binds to TROP2-positive tumors, penetrates cells, and releases the microtubule inhibitor MMAE to induce tumor cell death. Structurally, AST-203 is composed of an anti-TROP2 antibody linked to the cytotoxic microtubule-inhibiting payload MMAE via a VC-PAB linker. MMAE is the same payload used in Padcev, the ADC therapy developed by Astellas and Seagen. ADC therapy TROP2 acts as an intracellular calcium signal transducer involved in cell proliferation and survival. Among TROP2-targeting new drugs, only two commercialized products exist: Gilead's ADC Trodelvy and Daiichi Sankyo/AstraZeneca's Datroway. Both products have only secured indications for breast cancer. Because TROP2 is highly expressed in breast, non-small cell lung, colorectal, and pancreatic cancers, the later entrants have been actively conducting clinical studies to tackle such major solid tumors. Aptamer Sciences is leveraging its proprietary ADC platform, “Aptamer,” to address the limitations of conventional ADCs. Aptamers are roughly one-tenth the size of antibodies, enabling deeper penetration into tumor tissue and faster delivery to target cells, enhancing therapeutic efficacy. In preclinical studies using tumor spheroid models (three-dimensional cultured cell aggregates), AST-203 demonstrated a 6.7-fold higher tumor penetration rate compared with Trodelvy. Prestige Biopharma has entered a clinical trial in the U.S for its antibody drug candidate PBP1510. PBP1510neutralizes the PAUF protein, a key driver overexpressed in pancreatic ductal adenocarcinoma (PDAC) that is a major target in pancreatic cancer. Through its Phase I trial, the company aims to assess the safety and tolerability of PBP1510 in combination with gemcitabine. NeoImmuneTech's NT-I7 received FDA orphan drug designation this year for pancreatic cancer. NT-I7 is an anti-cancer drug candidate targeting IL-7, which organizes T-cell development and function, and targets various indications. Immuno-oncology drugBeyond pancreatic cancer, NT-I7 previously received ODD from the FDA for CD4 lymphopenia (2019), progressive multifocal leukoencephalopathy (2020), and glioblastoma (2023). NeoImmuneTech is currently conducting a Phase II trial evaluating NT-I7 in combination with Keytruda. The trial enrolled 50 patients with previously treated metastatic colorectal cancer and 48 with pancreatic cancer. Interim data showed partial responses (PRs) in three of the 48 pancreatic cancer patients, with a median overall survival (OS) of 11.1 months.
- Company
- Co-promotion discussed for the diabetes drug Mounjaro
- by Kim, Jin-Gu Aug 27, 2025 06:07am
- Product photo of Mounjaro The possibility of a co-promotion partnership between Eli Lilly and Korean pharmaceutical companies for 'Mounjaro,' the treatment of diabetes and obesity, continues to be discussed. While Lilly Korea officially maintains a 'direct sales' policy, it is reported that they are also conducting undiscloed discussions for potential partnerships. It is anticipated that the decision on co-promotion will become clearer around the time Mounjaro becomes reimbursed for the diabetes indication. According to industry sources on August 26, Lilly Korea recently discussed the possibility of co-promoting Mounjaro with approximately ten domestic pharmaceutical companies. Key major pharmaceutical companies with nationwide sales networks are said to have participated in the discussions, exchanging ideas by proposing their sales strategies to Lilly Korea. However, Lilly Korea's official position of 'direct sales' remains unchanged. An official from Lilly Korea stated, "At the time of launch, Lilly will handle direct sales, and distribution will primarily be through distributors who have direct dealings with Lilly." In other words, it is interpreted as a move to maintain the principle of direct sales while leaving the door open for collaboration with domestic pharmaceutical companies through simultaneous undisclosed discussions. The pharmaceutical industry believes that Mounjaro's 'indications' are the reason why two sales models are being considered simultaneously. Mounjaro currently holds three indications: ▲Improving glycemic control in adult patients with Type 2 diabetes ▲Chronic weight management in adult patients ▲Treating moderate-to-severe obstructive sleep apnea in obese adults. With patient groups categorized for diabetes and obesity, the sales strategy must be differentiated. This situation differs from the global market. In the U.S., the same active ingredient (tirzepatide) is marketed separately as an obesity drug called 'Zepbound' and a diabetes drug called 'Mounjaro.' In Korea, however, it was approved as a single product under the name Mounjaro, without using the name Zepbound. Mounjaro is therefore sold as both an obesity and a diabetes drug. The consensus in the industry is that discussions regarding a co-promotion partnership with domestic pharmaceutical companies are focused on the diabetes treatment indication. It is reported that the diabetes indication was a key topic of discussion during the negotiation process. The pharmaceutical industry anticipates that Lilly Korea will pursue reimbursement for Mounjaro's diabetes treatment indication, targeting the first half of next year. If reimbursement is approved, it would be possible to separate the sales strategies for the diabetes and obesity markets. This means that Mounjaro for obesity treatment could be sold exclusively by Lilly Korea's in-house sales team. At the same time, a co-promotion system could be established with a domestic pharmaceutical company for Mounjaro's diabetes treatment. Consequently, the co-promotion partner is expected to be known around the time reimbursement is secured. The high market potential of Mounjaro is the reason for the strong interest from Korean pharmaceutical companies. Mounjaro achieved record-breaking sales in the global market immediately after its launch. As a diabetes drug, its globalsales reached $5.198 billion (approximately KRW 7 trillion) in the second quarter of this year. Zepbound, initially launched as an obesity drug, generated $3.381 billion (approximately KRW 4.7 trillion) in sales. In just three months, the combined sales of the two products amounted to nearly KRW 12 trillion. Considering Mounjaro's significant presence in both the diabetes and obesity markets, it is likely that Korean pharmaceutical companies will find it difficult to pass up this strategic opportunity. A co-promotion partnership could immediately lead to external growth and a synergistic effect with their existing diabetes treatment portfolios. The high potential for future indication expansion beyond diabetes and obesity is also an attractive factor. Currently, GLP-1 class drugs like Mounjaro and Wegovy are being actively investigated in clinical trials for conditions such as MetabolicDysfunction-Associated Steatohepatitis (MASH), cardiovascular diseases, and kidney diseases.