- LOGIN
- MemberShip
- 2025-12-18 22:52:17
- Policy
- MOHW Minister Jeong 'Will expedite orphan drug listings'
- by Jung, Heung-Jun Oct 16, 2025 06:20am
- The Ministry of Health and Welfare announced it will support the expedited listing of new drugs to enhance access to rare disease treatments and develop tailored measures for each rare disease. It also plans to collaborate with the Korea Disease Control and Prevention Agency to expand the legal designation of rare diseases. During the National Assembly Health and Welfare Committee audit held on October 14, Rep. Gae-ho Lee of the Democratic Party of Korea urged the ministry to take measures to strengthen support for rare diseases. Rep. Lee said, “Around 7,000 rare diseases have been identified worldwide, but only 1,314 are legally recognized as rare diseases in Korea. Patients also face significant challenges in accessing new therapies, and coverage under the national insurance system remains limited,” he added, calling for a comprehensive review by the ministry. In response, Minister Jeong stated, “We are well aware that rare diseases are marginalized, leading to insufficient access to new drugs and inadequate welfare support. We conduct annual demand surveys to expand the list of rare diseases and will consult with the Korea Disease Control and Prevention Agency to ensure this process is conducted meticulously.” She added, “We will also ensure new drugs can be listed promptly. Since medical and welfare needs vary by rare disease, we believe strengthening tailored countermeasures is necessary and will actively pursue this.”
- Policy
- "Overseas transfer of KOR genomic data by China's Novogene"
- by Lee, Jeong-Hwan Oct 16, 2025 06:20am
- During the Parliamentary Inspection, Rep. Lee Ju-young questioned the possibility of Novogene Korea transferring Korean citizens Rep. Lee Ju-young of the Reform Party pointed out an issue regarding Novogene, a Chinese genomics analysis company that established a branch in Korea, that the company has no genetic analysis equipment in Korea. Rep. Lee's concern is that Novogene's holding company, BGI, was globally reported in 2021 for allegedly collecting and sharing genomic data from over 8 million pregnant women across 52 countries with the People's Liberation Army. This raises serious concerns regarding the export of domestic genomic information and poses a threat to national security. Furthermore, Novogene Korea is located in a building owned by the Korea Association of Health Promotion, where the tenancy rights reportedly include the authority to use the Association's big data. This suggests an increased risk that Novogene could directly pose a national security threat by exporting Korean citizens' genomic data. Rep. Lee suggests that the current 'Bioethics and Safety Act' does not regulate Novogene Korea's reporting, which has triggered these concerns. She urged the Ministry of Health and Welfare (MOHW) to prepare responses. Minister of Health and Welfare Jeong Eun Kyeong stated that she would examine the facts regarding the sharing of domestic genomic big data between Novogene Korea and the Association. On October 14, during the parliamentary inspection of MOHW, Rep. Lee questioned Minister Jeong regarding the overseas export of domestic patient genomic data by Novogene Korea and the related threat to national security. According to Rep. Lee, Novogene Korea has not been registered as a genetic testing institution because the current law does not require such registration. As a result, the company operates as a sales organization with only six employees without the necessary equipment for genomic analysis. Rep. Lee criticized this, arguing that it makes it inevitable that genomic samples collected domestically by Novogene Korea will be sent to the company's centers in Mainland China, Hong Kong, and Singapore for analysis. It may be concluded that domestic biological genomic information is being exported. Rep. Lee said, "Institutions like Novogene Korea, which perform research and not testing for purposes defined by the Bioethics and Safety Act, are not subject to reporting as a genetic testing institution," and added, "The legislative gap or regulatory blind spot was created when the regulation mandating reporting for research-purpose testing companies was deleted in 2013." Rep. Lee also pointed out, "Current law does not restrict the export of biospecimens for genetic analysis or limit the performance of genetic testing overseas," and added, "Even if there is a concern that genetic testing prohibited domestically might be carried out overseas or that domestic genomic information might be exported, there is no legal basis to sanction it." She further pointed out, "Novogene Korea is located in the Korea Association of Health Promotion building. The arrangement appears to be a co-sharing lab model with the tenant. The company has no equipment or personnel for genetic analysis or experimentation. It has six employees, all of whom are administrative or sales staff, and the CEO does not even reside in Korea." Minister Jeong Eun Kyeong promised to verify the allegation.Rep. Lee asked, "I requested the attendance of Novogene Korea's witness, but they submitted a statement of non-attendance. The Association's national genomic big data is clean data of the Korean people. It is highly valuable and risky if leaked, directly affecting the national security of the Republic of Korea. When were you briefed on this?" Minister Jeong replied, "I received a report confirming that Novogene Korea and the Association have a simple lease agreement and no history of information sharing or joint research," and added, "We need to verify how the Association's big data is being shared." Minister Jeong further said, "I will verify the details of whether Novogene Korea receives requests for genomic information analysis from other Korean hospitals or institutions and then sends those samples overseas for testing."
- Company
- ‘Leclaza combo extends survival without chemotherapy’
- by Son, Hyung Min Oct 15, 2025 11:37am
- Professors Sun Min Lim and Byoung Chul Cho of Yonsei Cancer Center’s Department of Medical Oncology “The MARIPOSA study is the first clinical trial to significantly extend overall survival using two targeted therapies without chemotherapy. Discussions on EGFR-mutated lung cancer treatment will now shift to focus on overall survival.” During a recent interview with Dailypharm, Professors Byoung Chul Cho and Sun Min Lim of Yonsei Cancer Center’s Department of Medical Oncology expressed so regarding the results of the MARIPOSA trial that evaluated the combined use of Leclaza (Lazertinib) and Rybrevant (amivantamab)’, agreeing it will trigger a paradigm shift in treatment. Leclaza (lazertinib), developed by Yuhan Corporation, is a third-generation EGFR tyrosine kinase inhibitor (TKI) that targets exon 19 deletions and exon 21 L858R mutations in EGFR-positive non-small cell lung cancer (NSCLC). Johnson & Johnson secured global rights to Leclaza and has conducted clinical studies evaluating the efficacy of Leclaza in combination with Rybrevant, a targeted therapy option targeting exon 20 and MET mutations. In the trial, the Leclaza + Rybrevant group demonstrated a statistically significant improvement in survival duration compared to the Tagrisso (osimertinib) group (p-value less than 0.005). Specifically, the median overall survival (OS) for the Leclaza + Rybrevant group was not reached (42.9-NE). In contrast, the Tagrisso group showed an OS of 36.7 months. Considering the survival rate distribution between the two groups, the Leclaza + Rybrevant group is expected to extend OS by at least 12 months compared to the Tagrisso group. These study results were recently published in the New England Journal of Medicine (NEJM), drawing significant attention from the academic community. Professor Cho explained, “The MARIPOSA study is the first clinical trial to significantly extend survival using only two targeted therapies without chemotherapy. While discussions on EGFR-mutated lung cancer have previously focused on progression-free survival (PFS), the focus will now shift to OS.” Professor Lim also stated, “In actual clinical settings, the third-generation TKI combo regimen showed rapid response and early symptom relief. If initial side effects are appropriately managed and prevented, treatment can be sufficiently continued on an outpatient basis. Ultimately, helping patients survive long-term with effective drugs is the top priority of treatment.” The professors saw that the most significant distinguishing feature of this study was the confirmation of a ‘qualitative change in the mechanism of resistance.’ Professor Byoung Chul Cho of Yonsei Cancer Center’s Department of Medical Oncology Professor Cho emphasized, “Not only did the frequency of resistance occurrence change, but the genetic and biological characteristics of the tumor itself changed. This suggests that the treatment fundamentally altered tumor biology, contributing to improved OS beyond merely extending the duration of treatment.” He further explained, “The MARIPOSA study demonstrated consistent OS improvement in both Asian and non-Asian patient populations. In contrast, the FLAURA2 study, which evaluated the efficacy of Tagrisso plus chemotherapy, showed a limited OS improvement in the Asian patient cohort.” He projected, “Combining chemotherapy has limitations in that it cannot fundamentally alter the biological characteristics of the tumor. Therefore, the detailed Asian data from MARIPOSA to be presented at ESMO Asia is expected to show different results compared to FLAURA2.” Professor Lim said, “In the detailed analysis of FLAURA2, no OS improvement was observed in the Asian cohort, excluding Chinese patients. Considering that the previous FLAURA study on Tagrisso monotherapy also failed to confirm OS improvement in Asian patients, racial differences may remain a persistent point of debate. Differences in drug response between races will become an important discussion point going forward.” Side effect management and formulation improvements increase potential for sustained treatment... “Combination therapy will ultimately become the standard” Researchers also note that recent studies have demonstrated manageability for skin-related adverse reactions, a common side effect of combination therapy. For Leclaza + Rybrevant, skin rash and paronychia are identified as major adverse reactions. Professor Cho stated, “According to the recently published COCOON study, preventive use of antibiotics, scalp lotions, and moisturizers reduced moderate to severe skin rashes by nearly half. If managed well during the initial 12 weeks, patients' quality of life also improves significantly.” Professor Lim emphasized, “We provide a management manual to patients and caregivers from the initial stages of treatment,” stressing that “prevention-focused management is key to ensuring treatment adherence.” Also, Professor Lim believed that the convenience of administration would significantly improve with the introduction of the subcutaneous injection formulation of Rybrevant. A drawback highlighted in the combination therapy of Leclaza + Rybrevant is that the injectable form of Rybrevant may reduce administration convenience. EGFR-targeted therapies, including Leclaza, Tagrisso, Boehringer Ingelheim's Giotrif (Afatinib), Pfizer's Vizimpro (dacomitinib), Roche's Tarceva (Erlotinib), and AstraZeneca's Iressa (gefitinib), are all oral medications. Rybrevant, however, is an intravenous (IV) formulation requiring a hospital visit once every three weeks for administration that lasts over an hour. This has raised concerns that it may hinder treatment convenience for non-small cell lung cancer patients. Janssen plans to maximize the synergy of combination therapy through the introduction of Rybrevant’s subcutaneous (SC) injection formulation. Professor Sun Min Lim of Yonsei Cancer Center’s Department of Medical Oncology Professor Lim stated, “In the PALOMA-2 study evaluating the potential of Rybrevant SC, the SC formulation showed infusion-related reactions reduced to one-seventh compared to IV, while demonstrating equivalent efficacy. Approval has already been granted in Europe, and U.S. approval is imminent. If introduced in Korea as well, the new formulation will significantly reduce the burden on patients.” She continued, “Beyond simply reducing administration time and increasing convenience, the SC formulation has the potential to alter the patient's immune environment itself. Our research team is currently preparing a study directly comparing the immunological differences between Rybrevant SC and IV administration.” She also noted, "The most important factor for patients is the survival period. As long as the data supports this, change in the field is only a matter of time. Delays in approval and reimbursement preventing patients from immediately benefiting from the latest treatments represent an institutional challenge that needs resolution.“ Professor Cho explained, ”While some hesitate to use combination therapy, citing concerns about Leclaza’s adverse reactions or reduced administration convenience, most issues can be resolved through dose adjustment and proactive management. The results of the PALOMA-2 and COCOON studies support this." He added, “The average age of EGFR-mutated lung cancer patients is mid-60s, about 10 years younger than the general lung cancer patient population. While caution is needed for combination therapy in those over 80, factors like treatment willingness, self-management capability, underlying conditions, and metastasis patterns are more critical criteria than age itself.” He stated, “If a patient has brain metastases but demonstrates strong treatment intent and good self-management capability, combination therapy should be prioritized. When Tagrisso first improved OS by six months eight years ago, some initially urged caution, but it eventually became the global standard. While some clinicians still prefer monotherapy, considering patient demand and the proliferation of data, combination therapy will likely establish itself as the new standard of care.”
- Company
- Will the MM drug Elrexfio be reimbursed this time?
- by Eo, Yun-Ho Oct 15, 2025 06:12am
- Whether progress will be made for the new multiple myeloma drug Elrexfio (elranatamab) in its second attempt to secure reimbursement in Korea is gathering attention. According to industry sources, Pfizer Korea's bispecific antibody therapy Elrexfio (elranatamab) is expected to be submitted for reimbursement review to the Health Insurance Review and Assessment Service's next Cancer Disease Review Committee. It remains to be seen whether Pfizer, which submitted a reapplication for Elrexfio’s reimbursement after its rejection by the HIRA Cancer Disease Review Committee last February, can achieve results within the year. Elerexfio previously received approval through a Global Innovative products on Fast Track (GIFT) designation by the Ministry of Food and Drug Safety.. Elrexfio is a fourth-line immunotherapy composed of two monoclonal antibodies - one targeting the antigen specific to multiple myeloma and the other engaging T cells. Bispecific antibody therapies are a form of immunotherapy composed of two monoclonal antibodies—one that recognizes a target antigen of multiple myeloma and another that binds to T cells. Typically, they are structured as bispecific IgG2 kappa antibodies that recognize BCMA (B-cell maturation antigen), the primary target antigen in multiple myeloma, and CD3. These therapies represent a novel approach that directly targets cytotoxic T cells to multiple myeloma cells expressing BCMA. Multiple myeloma, a cancer of plasma cells in the bone marrow, is a type of hematologic malignancy that primarily affects older adults. It is a disease where prolonged treatment can bring extended survival. Although various new therapies are being developed for the disease, monoclonal antibodies and bispecific antibody therapies are currently typically used in practice. In particular, the bispecific antibody mechanism is regarded as a safe and effective treatment for relapsed and refractory multiple myeloma, in which resistance increases with each treatment cycle, shortening the remission period and reducing the available treatment options. Since multiple myeloma is a disease where extended survival is achievable through continuous treatment, it is essential to have various therapeutic options available at each stage of treatment. This is why extending reimbursement coverage to fourth-line and later therapies remains an urgent priority. Currently, bispecific antibody therapies such as Elrexfio, Tecvayli (teclistamab), and Talvey (talquetamab) are approved in Korea, but none are granted reimbursement. Amid the failed discussions over coverage of a series of bispecific antibody drugs in the early stages, whether any drug will be granted reimbursement and improve patient access is gaining attention. Meanwhile, Elrexfio was designated by the Ministry of Food and Drug Safety as a GIFT item and was approved as a monotherapy for adult patients who have received more than three lines of treatment, including proteasome inhibitors, immunomodulators, and anti-CD38 monoclonal antibodies, in May last year. The US FDA has also designated it as a breakthrough therapy and granted accelerated approval for the drug. Elrexfio’s efficacy was demonstrated through the Phase II MagnetisMM-3 trial, an open-label, multicenter, non-randomized study that was conducted on 123 patients who had not received prior BCMA-directed therapy (i.e., BCMA-naïve patients). Results of Cohort A showed that the drug recorded an objective response rate (ORR) of 61.0% and a complete response (CR) of 37.4%. The progression-free survival (PFS) period was 17.2 months, and the overall survival (OS) period was 24.6 months, demonstrating an unprecedented long-term treatment effect. The data demonstrated that Elrexfio provided long-term survival benefits and slowed down disease progression to improve the quality of life of patients who had no other treatment options.
- Opinion
- [Op-Ed] Patients, no time left for 'new drug comb therapies'
- by Eo, Yun-Ho Oct 15, 2025 06:11am
- Eunyoung Lee, Board of Director of the Korea Alliance of Patients Organization"My father (in his 60s), who has been diagnosed with urothelial carcinoma, is paying 10 million won every three weeks for combination cancer therapy that is non-reimbursed. The disaster medical expense program provided the cost. However, we feel helpless upon the news that the fund is being delayed." This is part of a public complaint filed at the Korea Alliance of Patients Organization. This case reflects the current situation in which access to new drug treatments depends on individuals' financial ability. In April, the government amended part of the National Health Insurance criteria for combinations of anticancer therapy. It was intended to correct an unjust system in which previously reimbursed pharmaceuticals are no longer covered when used in combination with a new, non-reimbursed drug. The Patients Organization and related academics have long demanded this, and it was a significant change that improved access to treatment. The combination therapy containing urothelial treatments 'Padcev (enfortumab)' and 'Keytruda (pembrolizumab)' is the key example. The Padcev + Keytruda combination therapy received approval from the Ministry of Food and Drug Safety in July 2024 as a first-line treatment indication. The clinical trial results demonstrated that the Padcev + Keytruda combination therapy reduced the mortality risk by 53% compared to the existing chemotherapy and extended overall survival by over 2-fold. The results were presented at ASCO GU 2024. In particular, the Padcev combination therapy is already reimbursed in six of the A8 countries whose drug prices Korea references: the United States, the United Kingdom, France, Germany, Japan, and Canada. The United Kingdom is operating the 'Combination Therapy Framework' to enhance patient accessibility. Furthermore, it reportedly allows swift discussion of reimbursement for new drugs developed by different pharmaceutical companies when there is sufficient clinical evidence. However, in Korea, the combination therapy was considered by the Cancer Disease Review Committee (CDRC), but it failed to set reimbursement criteria. It is reportedly anticipated to be reconsidered for CDRC in October. It means that no discussion has yet begun. During this time, patients paid high-priced, non-reimbursed treatment out of pocket. Several patients have given up on receiving treatment. The Padcev + Keytruda combination therapy for one month costs from approximately million won to 10 million won. Urothelial carcinoma is highly likely to relapse and metastasize, and it has only a few treatment options. Despite the clinical efficacy of a combination therapy, the evaluation and negotiation process is complicated for new combination drugs developed by different pharmaceutical companies. Therefore, the reimbursement discussion is being delayed. Whereas the government took the first step toward 'combination with existing reimbursed pharmaceutical and new drug' with the amendment in April, an institutional basis must now be established so that 'combination of new drug-new drug' is reasonably evaluated and discussed. There must be a procedure for reviewing the clinical value of combination therapies and for guiding collaborative models between companies. As the discussion on new drug-new drug combination therapy is being delayed, there are fewer treatment opportunities for patients, and treatment options become more limited. Patients sincerely wish for opportunities to access the currently available treatment, not the launch of new drugs. We wish that clinically proven combination therapies were swiftly and reasonably evaluated. The current situation in which systemic procedures and structural limitations triumph over patients' willingness to continue treatment, and the treatment effectiveness must be improved.
- Policy
- New Divisions for Essential & Public Healthcare proposed
- by Lee, Jeong-Hwan Oct 15, 2025 06:11am
- Ministry of Health and Welfare Minister Eun-kyeong Jeong The Ministry of Health and Welfare has decided to establish a new director-level organization, the ‘Regional, Essential, and Public Healthcare Policy Office,’ to ensure the success of the Lee Jae-myung administration's national policy task of people-focused healthcare reform. It has been confirmed that this request has been submitted to the Ministry of Interior and Safety as a top priority. This effectively kicks off the organizational restructuring process, where the existing ‘Healthcare Policy Office’ under the Second Vice Minister will handle legislative and policy-based tasks, while the new ‘Regional, Essential, and Public Healthcare Policy Office’ will focus on practical healthcare reform initiatives such as the regional doctor system, public medical schools, and telemedicine projects. Alongside the separate establishment of the Regional, Essential, and Public Healthcare Policy Office, the Ministry also conveyed to the Ministry of the Interior and Safety its request for the creation of a director-general level organization (second priority) dedicated to the ‘Integrated Support Act for Regional Care, including Medical and Nursing Care,’ set to take effect next March, and the establishment of a director-level ‘Pharmaceutical and Bio Industry Policy Office’ (third priority). According to National Assembly and Ministry of Health and Welfare officials on the 14th, the Ministry is considering an organizational restructuring plan targeting successful healthcare reform, the smooth implementation of systems related to the Integrated Care Act, and the promotion of the domestic pharmaceutical and bio industry. The Ministry has conveyed its request to the Ministry of the Interior and Safety for the creation of a director-level organization and a bureau-level organization. It plans to accelerate the administrative process for organizational restructuring and expansion based on the Ministry of the Interior and Safety's decision. Currently, under the Second Vice Minister of Health and Welfare, there is one Office — the Healthcare Policy Office — and three Bureaus: the Health Insurance Policy Bureau, the Health Policy Bureau, and the Health Industry Policy Bureau. Additionally, three temporary organizations—the National Pension Reform Support Team, the Biohealth Innovation Promotion Team, and the Medical Reform Promotion Team—are operating separately. The Healthcare Policy Office is responsible for government legislative and administrative tasks concerning health and medical policy and health and healthcare finance overall. Ministry of Health and Welfare Minister Eun-kyeong Jeong has emphasized the need for a dedicated unit focused on regional, essential, and public healthcare operations while maintaining the existing policy-based Healthcare Policy Office structure. The rationale is that for the Lee administration to properly succeed with essential healthcare strengthening policies—which the previous Yoon administration failed to unilaterally implement, including plans to increase medical school enrollment by 2,000—a dedicated director-level organization for regional, essential, and public healthcare operations would be essential. Currently, the Ministry has the ‘Medical Reform Promotion Team,’ a temporary director-level organization established during the Yoon administration. Led by Director Gyeong-sil Jeong, this team is handling the transfer of related duties to the Public-Focused Medical Reform Committee following the launch of the Lee administration. Minister Jeong is expected to abolish this temporary team and establish the new director-level Regional, Essential, and Public Healthcare Policy Office. Furthermore, Minister Jeong reportedly holds the view that the Healthcare Industry Policy Bureau should be elevated to a director-level Pharmaceutical and Bio-Industry Policy Office to properly foster the domestic pharmaceutical-bio industry and biohealth industry. She has also asked MOIS to elevate the temporary unit under the Senior Policy Office for Population and Social Services, which currently handles elderly policy, into a full-fledged “Integrated Care Bureau for Medical, Nursing, and Community Services.” As strengthening regional, essential, and public healthcare, fostering a globally competitive pharmaceutical and biotech industry, and ensuring a smooth landing for a regionally integrated care system are all presidential campaign pledges and national policy tasks, attention is focused on how much the Ministry of the Interior and Safety will accommodate the Ministry of Health and Welfare's requests. Multiple officials from the National Assembly and the Ministry of Health and Welfare agree on the necessity of establishing a separate Regional-Essential-Public Policy Office alongside the Healthcare Policy Office. The prevailing view is that the Ministry of the Interior and Safety must also accept the establishment of a Pharmaceutical and Bio Industry Policy Office and the elevation of the Integrated Care Bureau to successfully advance these national policy tasks. An MOHW official stated, "The Healthcare Policy Office will operate as a policy and legislative foundation organization, while the Regional-Essential-Public Policy Office handles comprehensive administrative tasks by sector. We see no overlap or conflict in their duties. If the Regional-Essential-Public Policy Office takes charge of practical tasks related to regional healthcare, public medical schools, and advancing the healthcare delivery system, the work will be clearly separated. Other ministries, such as the Ministry of Trade, Industry, and Energy, also have director-level organizations structured as Policy Division, Infrastructure Division, and Business Division."
- Company
- GSK’s myelofibrosis drug Omjjara faces reimb hurdles
- by Eo, Yun-Ho Oct 14, 2025 06:41am
- The reimbursment of GSK’s new myelofibrosis drug Omjjara has become uncertain in Korea. According to Dailypharm coverage, the reimbursement application for GSK Korea's myelofibrosis treatment Omjjara (momelotinib), which had passed the Cancer Disease Deliberation Committee of the Health Insurance Review and Assessment Service (HIRA) in March, was not placed on the agenda of the Drug Reimbursement Evaluation Committee. It appears that differences arose between GSK and HIRA over the selection of a comparator drug used for pricing, effectively halting Omjjara’s first reimbursement attempt. As a result, it remains to be seen whether GSK will resubmit a reimbursement application and proceed with the listing process. Omjjara has a triple mechanism of action that inhibits JAK1 and JAK2 as well as ACVR1 (activin A receptor type 1). In myelofibrosis treatment, JAK1 and JAK2 inhibition helps relieve systemic symptoms and reduce splenomegaly, while ACVR1 inhibition decreases hepcidin expression and thereby alleviates anemia. Anemia management remains one of the major unmet needs in treating myelofibrosis. Transfusion-dependent anemia brings more than just the commonly perceived issue of dizziness - depending on its severity, it can be life-threatening. Phase III trials SIMPLIFY-1 and MOMENTUM demonstrated that Omjjara significantly improved key symptoms such as splenomegaly and reduced transfusion dependence in anemic myelofibrosis patients regardless of prior JAK-inhibitor exposure. In SIMPLIFY-1, which compared Omjjara to ruxolitinib (Jakavi) in JAK-inhibitor-naïve myelofibrosis patients, Omjjara demonstrated non-inferiority to ruxolitinib in the primary endpoint of spleen volume response at week 24. The proportion of transfusion-independent patients was 66.5 % in the Omjjara group versus 49.3 % in the ruxolitinib group, showing a statistically significant reduction in transfusion dependence in the Omjjara arm. Professor Seo-yeon Ahn of Chonnam National University Hwasun Hospital’s Department of Hematology stated, “Existing JAK inhibitors relieve splenomegaly and systemic symptoms but often worsen anemia or increase transfusion needs, leaving an unmet clinical need. Omjjara demonstrated significant clinical value in improving anemia, which is closely tied to prognosis in myelofibrosis patients.”
- Company
- Hugel appoints Carrie Strom as global CEO
- by Chon, Seung-Hyun Oct 14, 2025 06:41am
- Hugel announced on October 13 that it has appointed Carrie Strom, a medical aesthetics leader and a former Senior Vice President at AbbVie, as its new Global CEO. Carrie Strom, HugelCarrie Strom will lead Hugel's global business operations. She is highly regarded as an expert in the aesthetics field, having served for five years, from May 2020 to February this year, as the Senior Vice President at the global pharmaceutical company AbbVie and the President of Allergan Aesthetics Global. Strom initially joined Allergan (now AbbVie) in 2011 and has since led the aesthetics portfolio, which includes the botulinum toxin product 'Botox' and the HA filler 'Juvéderm,' across more than 50 countries. Previously, she served as Senior Vice President of Allergan's U.S. Medical Aesthetics division and spent 11 years as a sales and marketing expert at Pfizer. Following the recent appointment of Jang Doo-hyun as the Chief Executive Officer for the Korean operations, Hugel plans to accelerate its growth, particularly in the Americas, with the addition of the new Global CEO. Carrie Strom stated, "I am excited to work with Hugel's talented and dedicated employees and Board of Directors," and added, "My top priority will be to build upon Hugel's leadership in Korea to become a global leader in the aesthetics market, raising the standards of service we provide to customers and patients worldwide." Suk-yong Cha, Chairman of Hugel's Board of Directors, stated, "CEO Carrie Strom is an expert who has gained extensive experience and driven change in the global medical aesthetics industry. We expect her to maximize Hugel's future value during this critical transition to a global enterprise, particularly in the Americas."
- Product
- KMA forms special committee to block INN prescribing
- by Kang, Shin-Kook Oct 14, 2025 06:41am
- KMA President Taek-woo Kim The Korean Medical Association (KMA) has formed a special countermeasure committee to block the institutionalization of international non-proprietary name (INN) prescribing. This is seen as a response to demands by some delegates for an extraordinary general meeting. KMA (President Taek-woo Kim) said on the 13th, “Policies such as INN prescribing and changes to laboratory test consignment rules are being unilaterally promoted despite threatening patient safety.” He announced plans to launch a Pan-Medical Countermeasure Committee for National Health Protection (tentative name, “Countermeasure Committee” and to hold a nationwide delegates meeting at 5 p.m. on the 25th in the KMA hall’s main auditorium to unite the opinion of medical organizations. KMA argued, “INN prescribing is a system where only the generic name of the drug is written instead of the brand name prescribed by the physician, allowing pharmacists to arbitrarily substitute and dispense medications. This infringes upon the physician's medical judgment, which is the core of personalized prescriptions issued for patient treatment, and poses serious systemic risks threatening public health by causing drug side effects and treatment confusion.” It further contended, “The primary causes of the instability in drug supply mentioned in the proposed bill lie in various structural problems, such as the government's unilateral drug pricing structure, insufficient production lines at pharmaceutical companies, and shortages in raw material supply. This refers not just to the unstable supply of a single specific brand-name drug, but to the scenario where the supply of all drugs with the same ingredient (due to raw material shortages, etc.) is interrupted. Addressing this with INN prescribing is a completely illogical idea.” The KMA emphasized, “Choosing the dangerous and misguided method of INN prescribing while ignoring the fundamental problems causing drug supply instability is a declaration of abandoning public safety and lives.” Furthermore, the KMA strongly opposed the proposed changes to unilateral revision of laboratory-testing consignment rules, calling it an attempt to eliminate essential and primary medical care. The KMA stated, “The specimen testing outsourcing system being unilaterally pushed by the government is a one-sided measure that ignores the realities of the medical field. It is a detrimental change that effectively paralyzes the diagnostic testing functions of medical institutions, safeguarding essential and primary care. In July 2023, the Ministry of Health and Welfare responded that it planned to consult sufficiently with the medical community regarding issues such as the lack of distinction between fees for outsourcing and consignment institutions when setting fees related to the notice on standards for laboratory-testing consignment rules. Yet, breaking this promise is pushing the policy forward unilaterally.” The KMA emphasized that it will gather the entire medical community's resolve through the formation of the committee and hold a national meeting of physician representatives, vowing never to back down and fight to the end.
- Company
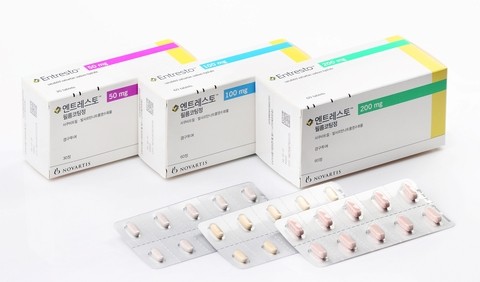
- Entresto patent dispute near its end, yet 0 generic approval
- by Kim, Jin-Gu Oct 14, 2025 06:40am
- Product photo of EntrestoThe patent dispute of Novartis' heart failure treatment, 'Entresto,' is nearing its final judgment. Still, generic companies may be unable to launch their products early, regardless of the ruling. To launch a generic early, companies need not only to win the patent dispute but also to secure product approval from the Ministry of Food and Drug Safety (MFDS). The MFDS is reportedly demanding supplementary data from the relevant generic companies. In other words, generic manufacturers, after fighting patent battles for five years, risk winning the legal fight only to be unable to launch their product. Not a simple combination drug but a 'cocrystal complex'...may be delaying generic approval According to the MFDS on October 13, there are currently zero product approvals for Entresto generics. This is considered unusual given that generic product approvals are typically processed within a year and a half of application. Approximately 10 generic companies involved in the Entresto patent dispute applied for generic product approval sequentially from April 2022 to July of the following year. They filed these applications based on their first-instance victory in the patent dispute. However, there has been no news of Entresto's generic product approval for almost 3 years. It is reported that the MFDS is requesting supplementary approval documentation. The pharmaceutical industry is paying attention to the MFDS's request for supplementation regarding Entresto's unique crystal structure. Entresto is a heart failure treatment in which the active ingredients, sacubitril and valsartan, act on cardiac neurohormones through separate pathways. The unique feature is that the two ingredients form a single crystalline structure, a cocrystal complex. Medicines combining two or more ingredients typically involve a simple mixture of the crystalline forms of each component. In contrast, a cocrystal involves two or more components bound together at the molecular level like a single compound. They exhibit single-compound properties until just before absorption into the body. For this reason, the industry refers to Entresto not merely as a 'combination drug' but as a 'complex' with a singular characteristic. The problem is the scarcity of approved generics for this cocrystal formulation. Entresto is reportedly the only drug approved as an API-API cocrystal complex, not only in Korea but also in the U.S. and Europe. Furthermore, there is no precedent anywhere in the world for the approval of a generic for a cocrystal complex drug. The MFDS is contemplating this issue. The Ministry is struggling to find an appropriate analytical method for generic approval. Critics suggest that because the cocrystal structure differs from traditional combination products in its physicochemical properties, applying the same analytical methods may not be suitable for generic approval. A pharmaceutical industry official said, "If it were a typical combination product's crystal form, there would have been no issue with approval, but I understand the MFDS is deeply concerned about whether it is appropriate to use existing methods to analyze this special cocrystal structure and grant generic approval." Winning in the Supreme Court, yet generic companies may face a failed launch Given the circumstances, companies preparing Entresto generics are now in a position where they may be unable to launch their products even if they win the final patent dispute. Generic companies initiated a declaratory judgment action against Novartis's Entresto crystal form patent in January 2021. This was followed by the filing of invalidation and circumvention trials against the use patent, salt/hydrate patent, and formulation patent. Generic companies won successive victories in the first and second instances. Disagreeing with these rulings, Novartis appealed the cases to the Supreme Court. Of the two cases appealed to the Supreme Court, the ruling in the use patent case was finalized in April of last year, with Novartis losing. The remaining pending case is the dispute over the crystal form patent. The full merits review is currently underway, following the dismissal without deliberation deadline in April of this year. Separately, the salt/hydrate patent dispute is awaiting a ruling from the Patent Court. The pharmaceutical industry reportedly anticipates that the final ruling regarding the crystal form patent could be issued as early as this year. If the Supreme Court sides with the generic companies, as in the first and second instances, the generic companies' patent risk would be effectively eliminated. However, regardless of the Supreme Court's decision, if MFDS approval continues to be delayed, generic companies will face a dual burden: winning a five-year-long dispute only to be unable to launch the product.