- LOGIN
- MemberShip
- 2025-12-18 22:52:14
- Policy
- “Cut prices of generic drugs across the board”
- by Jung, Heung-Jun Sep 29, 2025 06:07am
- A proposal has been made to establish reimbursement priority for treatments targeting rare/serious diseases versus mild conditions, thereby expanding access to new drugs. There were also calls to reform the distorted pharmaceutical budget structure by cutting generic prices across the board and canceling approvals for products lacking bioequivalence data. At a National Assembly forum on improving the operation of health insurance finances for rare and severe diseases, hosted on the 26th by lawmakers Mi-hwa Seo and Jong-tae Jang, participants stressed the need to allocate limited insurance resources more effectively to improve access to new drugs. Prof. Yong-jin Kwon of Seoul National University Hospital’s Public Healthcare Center Professor Yong-jin Kwon of Seoul National University Hospital’s Public Healthcare Center said, “The National Health Insurance system has reached a structural contradiction. While pharmaceutical spending exceeds the OECD average, access to innovative drugs remains restricted, depriving patients of treatment opportunities. Coverage for rare and severe diseases should have expanded, but instead, it has regressed.” Professor Kwon called for ▲Reestablishing reimbursement priorities, ▲reforming the drug management system , and ▲expanding access to new drugs. He explained that a survey conducted last July of 1,000 citizens showed significant public agreement on prioritizing coverage for patients with rare and severe diseases. According to the results, 46.7% agreed with prioritizing severe disease patients, and 52.7% supported prioritizing rare disease patients. However, 76.9% responded that while premiums should be maintained at the current level, coverage should be differentiated. Prof. Kwon said, “This suggests there is sufficient basis to regard reprioritization as being highly feasible.” Professor Kwon proposed the following improvement measures ▲establishing a National Health Insurance Priority Committee with public participation ▲implementing blanket price reductions for generic drugs and fostering competition, and ▲introducing a fast-track system for rapid listing and flexible benefit determination. Prof. Kwon said, “The share of pharmaceutical expenditures is already high, but the portion spent on new drugs is low. The generic market is driving distortion. We should consider whether this is due to high prescription volumes or high generic prices,” stressing the need to shift reimbursement priorities from mild to severe conditions. He added, “Prioritization should consider disease severity and social costs, as well as risks of treatment delays and potential market failures.” He also argued that generic drug prices should be cut across the board, and generics lacking bioequivalence testing should have their approvals revoked. He stated, “It is unworthy of our national standing that drugs without bioequivalence certification are still circulating. Bioequivalence test results should be made public. Citizens must be convinced that the efficacy and composition are identical.” Furthermore, he called for expanding fast-track reimbursement and flexible decision-making for rare disease drugs. Suggestions included accelerating reviews, granting conditional approvals, and enhancing post-marketing management through fast-track pathways. He concluded, “We must streamline the health technology assessment process and implement conditional approvals. If MFDS approval represents the product, insurance reimbursement represents the technology. Even if not yet officially authorized, reimbursement should be possible based on technological appropriateness. For rare diseases, conditional approval and reimbursement must be adopted, and real-world evidence (RWE) should be leveraged for more sophisticated post-market management.” He added, “Flexible reimbursement frameworks must be expanded. Risk-sharing agreements (RSA) should be applied diversely, such as on a performance-based basis. Patients should also understand that the limited coverage can be gradually expanded over time.”
- Company
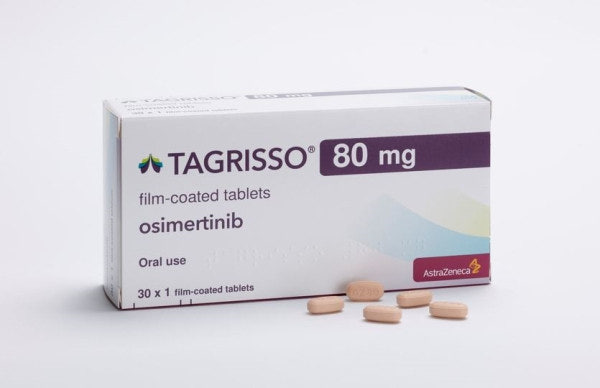
- CKD and Kwang Dong win Tagrisso formulation patent suit
- by Kim, Jin-Gu Sep 26, 2025 06:14am
- Generic drugmakers challenging AstraZeneca's patent for the non-small cell lung cancer treatment ‘Tagrisso (osimertinib)’ have won their case in the first instance. According to industry sources on the 25th, the Intellectual Property Trial and Appeal Board (IPTAB) on the 24th ruled in favor of Chong Kun Dang and Kwang Donng Pharmaceutical in their request for a negative right scope confirmation trial regarding Tagrisso’s formulation patent (No. 10-2336378). This significantly increases the possibility of an early launch of Tagrisso generics. The two companies have also met 2 key requirements for obtaining exclusive marketing rights (first generic exclusivity): ▲ being the first to file the trial request and ▲ winning the trial/litigation. Now, they only need to satisfy the condition of being the first to file for generic approval to obtain the first generic exclusivity right. CKD and Kwang Dong are the only companies to have challenged the Tagrisso formulation patent. If both secure the first approval for their generics and gain the first generic exclusivity rights, they will be able to sell their product exclusively for 9 months. The key variable is AstraZeneca’s appeal. If AstraZeneca files a lawsuit to overturn the IPTAB decision with the Patent Court, the dispute may be prolonged. AstraZeneca's decision on whether to appeal has not yet been made. Tagrisso is protected by three patents: substance patents expiring in November and December 2033, and a formulation patent expiring in January 2035. CKD and Kwang Dong have successfully avoided the formulation patent. With the formulation patent out of the way, both companies plan to launch their generics as soon as the substance patent expires in 2033. There is also speculation that they could aim for an even earlier launch by targeting the extended duration granted to the substance patent. Tagrisso is a targeted anticancer drug used for EGFR-mutated non-small cell lung cancer (NSCLC) that received domestic approval in 2016. It is used as a first-line treatment for patients with locally advanced or metastatic NSCLC harboring EGFR gene mutations. In Korea, it competes with Yuhan Corp’s Leclaza (Lazertinib). According to the market research institution IQVIA, Tagrisso’s sales in Korea reached KRW 111 billion in 2023. After surpassing KRW 100 billion in annual sales in 2022, growth has continued steadily. With reimbursement expanded last year to include first-line treatment for locally advanced and metastatic NSCLC patients with specific EGFR mutations, sales are estimated to have exceeded KRW 130 billion. Separately, CKD is developing its own new drug for non-small cell lung cancer. The candidate compound, named ‘CKD-702’, is a bispecific antibody targeting both cMET and EGFR. It is currently undergoing global Phase I clinical trial. CKD is seeking to strengthen its foothold in the NSCLC treatment market by developing both a new drug and a generic.
- Opinion
- [Reporter’s View] The gap between innovation and access
- by Son, Hyung Min Sep 26, 2025 06:12am
- Advances in medicine have created pivotal turning points in the course of human survival. Diseases once deemed incurable are gradually being reclassified as chronic conditions. From anticancer drugs to treatments for rare diseases and immunotherapies, innovative new drugs have not only prolonged patients’ lives but also increased the burden of responsibility borne by nations and societies. The problem lies in the fact that the ‘pace of innovation’ and the ‘pace of patient access’ are not on the same track. Korean patients are always left to wait. Even when news arrives that a new drug has been approved in the US or Europe, a gap of 1 to 3 years often exists before patients can actually receive a prescription domestically. This delay is not merely a procedural issue; it represents a lost opportunity for treatment. For a terminal cancer patient, 1 year can be their entire life. This is why it's not uncommon for patients to seek treatment abroad. At the center of this irony lies a uniquely Korean formula: price over innovation. The value of a new drug is first calculated by its burden on the national health insurance budget rather than by its clinical significance and impact on patient survival. While managing insurance finances is clearly a crucial task in national governance, the balance has tilted excessively toward fiscal restraint, depriving patients of the opportunity to timely benefit from innovations. This is also why pharmaceutical companies often push Korea down the priority list in their global launch strategies. The so-called ‘Korea passing’ concern is not an abstract warning but a real risk that delays actual patients' treatment opportunities. The global environment is not favorable either. President Trump’s MFN (Most Favored Nation) drug pricing policy has posed a threat to pricing systems worldwide. Under the pretense of protecting American patients, it targeted countries like Korea that maintain low drug price systems as risk factors. From the perspective of multinational pharmaceutical companies, the incentive to launch drugs in Korea has diminished because protecting prices in the US market has become more critical. In other words, Korea’s low drug prices translate directly into being deprioritized in global market strategies. This leads us to a fundamental question: what is a new drug to the patient? To the government, it may be a budget variable; to companies, a profit variable. But to patients, a new drug is a survival variable. Unless the perspective shifts from “how cheaply can we bring it in” to “how quickly and fairly can it reach patients,” the meaning of innovation becomes powerless in front of patients. Of course, the constraints of the national health insurance budget are an undeniable reality. Within limited resources, it's difficult to unconditionally recognize the value of new drugs and raise their prices. The government isn't sitting idle either. New attempts are being discussed, such as the approval-evaluation linkage pilot project, expansion of risk-sharing schemes, and recently, indication-based pricing. But the fundamental limitations remain. Within the current system, patient access to new drugs inevitably remains low. A radical policy shift is now needed. So what constitutes as ‘radical’? Suggestions include introducing value-based assessment that prioritizes clinical innovation and patient survival outcomes; implementing multi-layered risk-sharing schemes where government, pharmaceutical companies, and society share burdens; and establishing a national strategy vision that enhances international negotiation power and improves patient access. Furthermore, a paradigm shift is needed—one that recognizes healthcare not merely as a fiscal issue, but as a core national competitiveness factor. Medical innovation will not cease. The problem is that the speed at which this innovation reaches Korean patients remains sluggish. How long can we tolerate the paradox of patients lagging behind in this era of innovation? Now is the time for government, industry, and society to find answers together. A decisive policy action to align the speed of innovation with patients’ needs is urgently required—so that patients no longer have to waste their time waiting.
- Company
- Teva-Handok challenges mkt with LAI risperidon, Uzedy
- by Hwang, byoung woo Sep 26, 2025 06:12am
- As the schizophrenia treatment paradigm shifts toward managing medication adherence, Teva-Handok has introduced Uzedy (risperidone), a long-acting subcutaneous containing risperidone, to the Korean market. Uzedy is expected to become a new treatment option, enhancing patient convenience with its 1-month and 2-month dosing options and a design that eliminates the need for a loading dose. This drug has been shown to reduce the risk of relapse by up to 80% compared to placebo. Uzedy logoTeva-Handok recently announced that its long-acting subcutaneous injectable for adult schizophrenia, Uzedy (risperidone), received approval from the Ministry of Food and Drug Safety (MFDS) on September 5. It is assessed as a new treatment option that overcomes the medication adherence issues associated with existing oral therapies and provides convenience for both patients and medical professionals. Notably, it is expected to contribute to securing 'treatment persistence,' which is critical in schizophrenia management. Uzedy is an long-acting injectable that can be administered at 1-month and 2-month intervals, developed specifically to address poor medication adherence, cited as the primary cause of schizophrenia relapse. Schizophrenia is a chronic, progressive mental illness affecting thought, emotion, and behavior, with about 80% of patients experiencing multiple relapses within the first five years of treatment initiation. Since repeated relapses can diminish treatment effectiveness, impair daily functioning, and even cause structural changes in the brain, consistent medication is essential. Uzedy's differentiation lies in its proprietary technology that eliminates the need for a separate high-dose initiation or oral supplementation during the initial treatment phase. Previous long-acting injectables required a high-dose loading dose or co-administration of oral medication for a certain period to reach therapeutic concentration levels. However, Uzedy is designed to achieve an effective blood concentration rapidly within 24 hours of administration using a special polymer technology, allowing for a fast onset of therapeutic effect. Professor A of Neuropsychiatry at a tertiary general hospital in Seoul explained, "The long-acting formulation can resolve the medication adherence issue where patients, due to low insight into their illness, stop taking their medication," and added, "Compared to daily oral medications, it maintains stable drug concentration in the blood, which is beneficial for maximizing therapeutic effect and minimizing the occurrence of side effects." Professor A also said, "This drug alleviates the patient inconvenience of having to take pills daily and the social burden of having to expose one's illness to others, thereby assisting with social reintegration and job retention." Risperidone, Uzedy's active ingredient, is a second-generation antipsychotic developed in the 1990s, with long-proven efficacy and safety in clinical settings. With the introduction of Uzedy, the schizophrenia long-acting injectable (LAI) market in Korea is expected to become a four-way competition. Following existing LAIs based on haloperidol decanoate (1st-generation), paliperidone (2nd-generation), and aripiprazole (3rd-generation), the addition of risperidone-based Uzedy is expected to provide diverse treatment options for schizophrenia patients. Given that some existing treatments offer longer dosing intervals than Uzedy, Uzedy's market establishment and competition will be linked to the overall therapeutic standing of risperidone. However, reimbursement listing is essential for Uzedy to expand its influence in the domestic schizophrenia treatment market. Teva-Handok said, "We have a plan for Uzedy's reimbursement and will follow the standard procedure for securing reimbursement after approval." Additionally, improving the perception of the LAI formulation, which currently has a low prescription rate in Korea, will be a challenge for Teva-Handok. Professor A said, "Research indicates that some clinicians avoid prescribing LAIs due to concerns that it might strain the patient-physician relationship or lead to criticism over forced injections," and stressed, "To increase the prescription rate, we need to eliminate this perception and for physicians to provide treatments they believe are necessary with a sense of responsibility." Hee Kyung Ahn, CEO of Teva-Handok, added, "Uzedy is expected to contribute to improving the quality of life for patients who struggle with long-term treatment due to low medication adherence by offering flexible dosing options and a rapid therapeutic effect simultaneously."
- Policy
- HIRA, "Will enhance the monitoring of rare disease drugs"
- by Jung, Heung-Jun Sep 26, 2025 06:12am
- With the rapid increase in newly emerging treatments for rare and severe diseases, there have been suggestions for establishing sustainable systems, such as strengthening post-market management and creating a separate dedicated fund. It was explained that monitoring uncertain evidence resulting from expedited approvals and improving patient access through a separate fund, similar to overseas models, are necessary steps. On September 25, the Health Insurance Review & Assessment Service (HIRA) hosted a symposium at the St. Mary's Hospital Seong-ui Hall in Seoul on the future direction and social ethics of rare and severe disease treatment. Lee So-young, Director of HIRADuring the symposium Lee So-young, Director of HIRA's Pharmaceutical Performance Assessment Division, said that as rare disease treatments rapidly increase, not only Korea but also other countries are deliberating on management strategies. In the United States, 52% of new drug approvals are for rare disease treatments, and the FDA operates four expedited tracks to address unmet medical needs. However, Lee pointed out that problems are arising where expedited approval, often based on surrogate endpoints, does not ultimately improve patient access or where research finds the drug provides no actual benefit. Lee explained, "The FDA is also aware of the problem. Since the year before last, they have required that when approval is based on surrogate index, a confirmatory clinical trial must be initiated or clearly planned," and added, "They have also reinforced measures to allow for rapid withdrawal of approval if the requirements are not met or if the anticipated clinical benefit is not realized." Lee emphasized that it is time for Korea to move beyond viewing rare disease treatment as an exceptional case and start considering a sustainable system. Lee proposed three strategies: ▲Conditional reimbursement based on evidence generation ▲Establishing a separate dedicated fund ▲Improving reimbursement criteria and pre-and post-market management. Regarding conditional reimbursement, Lee said, "The legal basis is established, and the guidelines will be released in November this year. This is a structure where a drug is listed and covered on the condition that post-market evaluation is conducted, and the post-market management tracks the extent of evidence uncertainty and whether that uncertainty can be eliminated." Lee added, "The UK classifies drugs into those that should or should not be listed through the Cancer Drug Fund. They have also established an Innovative Medicines Fund. Taiwan and Australia operate similar funds," proposing the establishment of a separate fund in Korea. Lee concluded, "Currently, reimbursement management is conducted at the patient level. There is a need for monitoring at the drug and disease level, with performance management based on those results,"and added, "It's difficult for pharmaceutical companies alone to gather and compile all the data. The EU also operates its system through transnational cooperation," and stressed the importance of a cooperative framework for strengthening post-market management.
- Policy
- Legislation of restricted INN Prescriptions gain momentum
- by Lee, Jeong-Hwan Sep 26, 2025 06:11am
- With the ruling party accelerating legislation of “restricted international nonproprietary name (INN) prescriptions,” which was one of President Jae-myung Lee’s campaign pledges, the medical community is showing visible unease. Democratic Party of Korea lawmakers In-soon Nam, Young-seok Seo, Yoon Kim, and Jong-tae Jang, along with Rebuilding Korea Party lawmaker Sun-min Kim, announced that they will host a policy forum on September 30 at the National Assembly under the theme “Introducing a Korean Model for INN Prescriptions.” In response, doctors’ associations have directly talked with Rep. Joo-min Park, chair of the NA Health and Welfare Committee, and are planning a protest rally. On the 24th, the Seoul Medical Association said it would hold a “Seoul Medical Association Representatives’ Rally Against INN Prescriptions” on September 26 at 7:30 a.m. at its headquarters. Participants will include the members of its executive board, auditors, the council’s steering committee, and presidents and executives of local medical associations. The day before, the Seoul Medical Association President Kyu-Seok Hwang visited NA Health and Welfare Committee Chair Joo-min Park's office on the 23rd for a meeting and conveyed their position on how the INN-based prescription policy threatens the sustainability of primary care. Doctors argue that since generic substitution is already legally guaranteed, further mandating INN prescriptions would jeopardize patient safety and undermine the foundation of Korea’s doctor-pharmacist separation system. They also contended that trying to address drug supply shortages through INN prescriptions is not a fundamental solution, and that the clause stipulating penalties of up to 1 year imprisonment or fines up to KRW 10 million is excessive. Nonetheless, the ruling party maintains the position that legislation is inevitable to resolve the harm to patients caused by shortages of essential and unstable supply of medicines. The lawmakers co-hosting the INN prescription forum with the Korean Pharmaceutical Association and the Korea Institute for Pharmaceutical Policy Affairs include not only pharmacist-turned-lawmaker Young-Seok Seo but also physician-turned-lawmakers Yoon Kim and Sun-min Kim, indicating broad support on the need for its institutionalization. The ruling party lawmakers recognize that the bill on restricted INN prescribing (led by Representative Jong-tae Jang), which is currently pending in the National Assembly, is far removed from undermining the intent of the separation of medical and pharmaceutical practices or threatening patient safety. This is because the bill proposes establishing a ‘Supply Management Committee for Medicines with Unstable Supply’ under the Ministry of Health and Welfare, involving both pharmacists and doctors, to designate out-of-stock drugs and permit generic prescribing only for those designated drugs. Lawmakers argue that legislation on restricted INN prescriptions must focus on easing patient inconvenience and improving access to medicines, rather than fueling conflicts over professional boundaries between doctors and pharmacists. A ruling party official said, “The restricted INN prescription bill should prioritize the public over doctors and pharmacists. The bill establishes a new committee to designate medicines with supply shortages subject to INN prescribing, and institutionalizes a management system requiring pharmaceutical companies, wholesalers, pharmacies, and physicians to submit information on such shortages. The goal is to resolve the long-standing inconvenience faced by the public.”
- Policy
- PVA monitoring for high-priced drugs conducted in Q4
- by Jung, Heung-Jun Sep 25, 2025 06:12am
- High-priced anticancer drugs, including Keytruda and Opdivo, have been placed under monitoring for price-volume agreements (PVA, Type A·B) in the 4th quarter. On the 23rd, the National Health Insurance Service (NHIS) released ‘prior information on the Q4 monitoring list for drugs subject to PVA (Type A·B).’ A total of 111 items were listed, including many blockbuster products from multinational pharma companies such as ▲ Novartis Korea’s Zolgensma (onasemnogene abeparvovec), ▲ MSD Korea’s Keytruda (pembrolizumab), ▲ Ono Pharmaceutical Korea’s Opdivo (nivolumab) 20mg, 100mg, 240mg, ▲ Roche Korea’s Alecensa (alectinib HCl) 150mg, ▲ Sanofi-Aventis Korea’s Dupixent (dupilumab) 200mg, 300mg. Also, products from Korean pharmaceutical companies on the list include: ▲ Celltrion’s Donerion Patch (donepezil) 87.5mg, 175mg, ▲ GC Biopharma’s Denol (bismuth subcitrate potassium), ▲ Bukwang Pharma’s Agio Granule, ▲ Dongindang Pharma’s LecClean Solution (sodium phosphate) 133ml, 1000ml, ▲ Ildong Pharmaceutical’s Pirespa Tab (pirfenidone) 200mg, and ▲ Boryung Corp’s Kanarb (fimasartan potassium trihydrate) 30mg, 60mg, 120mg. The NHIS said, “Claims filed for the monitored drugs will be analyzed, and if they meet the negotiation criteria, they will be designated as subjects for price-volume agreement negotiations.”
- Company
- Twice-yearly 'lenacapavir' for HIV expected to land in KOR
- by Eo, Yun-Ho Sep 25, 2025 06:12am
- Product photo of lenacapavir 'Lenacapavir,' a HIV prevention drug taken twice a year, is expected to be marketed in Korea. According to industry sources, the Ministry of Food and Drug Safety (MFDS) is currently reviewing the approval of lenacapavir from Gilead Sciences Korea. The drug was designated as an orphan drug in January. Its specific indication is "a combination therapy with other antiretroviral agents for the treatment of multidrug-resistant HIV-1 infection in adults who are not being treated with their current antiretroviral therapy." This drug, marketed overseas under the brand name Sunlenca, is the first long-acting HIV-1 capsid inhibitor and is administered as a subcutaneous injection every six months. It was approved in countries like the United States and Europe in 2022 and is currently being prescribed. Current HIV treatment is maintained through daily oral administration of antiretroviral drugs. However, with the development of long-acting formulations, the administration frequency is advancing to once every two months or once every six months. Lenacapavir is also garnering more attention for its potential in HIV 'prevention,' not just treatment. In June, this drug received approval in the U.S., and more recently in Europe, for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 infection in at-risk adults and adolescents weighing at least 35 kg. The product name for lenacapavir used in prevention is 'Yeytuo.' The efficacy of lenacapavir for prevention was proven through the Phase 3 PURPOSE 1 and PURPOSE 2 studies. The PURPOSE 2 study results showed that lenacapavir reduced HIV infection by 96% compared to the background HIV incidence (bHIV). In the study, 2 cases occurred among 2,180 participants, meaning that 99.9% of the lenacapavir arm did not contract HIV. Last year, Gilead also prematurely unblinded its PURPOSE 1 trial, which evaluated lenacapavir as PrEP in cisgender women in Sub-Saharan Africa, after it met its primary efficacy endpoint. Meanwhile, the international science journal and news Science selected lenacapavir as its 'Science Innovation of the Year' last year based on these research results.
- Company
- 'Drug pricing reform is overdue in Korea'
- by Son, Hyung Min Sep 25, 2025 06:11am
- KRPIA held a commemorative meeting marking its 25th anniversary on the 24th at Some Chavit in Banpo, Seoul. During the panel discussion held that day, experts raised the opinion that the industry and government must work together to improve patient-centered access to new drugs Concerns persist that domestic patients still face slow progress in accessing global new drugs. Although the government has introduced system reforms such as expansion of the risk-sharing scheme and pilot projects linking regulatory approval and reimbursement evaluation, experts argue that the current incentive system for innovative drugs is still insufficient, hindering both industry growth and patient treatment opportunities. On the 24th, the Korean Research-based Pharmaceutical Industry Association (KRPIA) held a roundtable to mark its 25th anniversary at Some Chavit in Banpo, Seoul. During the panel discussion, domestic and international pharmaceutical experts emphasized the need to improve patient-focused access to innovative drugs, pricing systems, and value recognition structures. Dong-ho Yeo from LG Chem, who has experience in both multinational and Korean pharmaceutical companies, stressed the importance of properly recognizing the value of innovative new drugs. Yeo said, “Although Korean companies don’t yet have many achievements that can truly be called innovation, they are continuing development. How the global innovative new drugs are evaluated will serve as an important signal for Korean companies as well.” “For Korean companies to move from being fast followers to first movers, innovative drugs must be appropriately valued. It would be difficult for a domestic drug to prove competitiveness in overseas markets if its innovativeness is not even recognized in its home country.” Panelists noted that despite systems being in place, it still takes a long time before actual patients gain treatment opportunities. They added that price negotiations and the financial savings logic often delay companies’ local launch strategies. Jae-min Cho, Senior Director at Eli Lilly Korea, said: “It’s encouraging that the government has designated biotechnology as a strategic national industry. However, the conservative value assessment practices of innovation hinder access for patients.” Cho pointed to ▲ Conservative evaluations that focus on price cuts rather than value recognition; ▲ Short evaluation periods (5–10 years) that fail to reflect long-term drug value; and ▲ even groundbreaking drugs developed after decades are often compared against the cost of outdated, low-priced drugs, as main challenges that hinder “Social consensus is needed on setting appropriate weighting factors and premiums that meet the Korean context. Only through continuous institutional improvements by the government, academia, and industry can we simultaneously achieve patient accessibility and industrial innovation.” Panels at the KRPIA 25th Anniversary Commemorative Panel Discussion “New drugs pushed out of priority lists... industry bears responsibility too” Responsibility within the industry itself was mentioned, alongside systemic limitations. Calls continued for the pharmaceutical industry to prioritize patient access as its foremost value. Dailypharm journalist Yoon-ho Eo remarked, “While the government and industry engage in reimbursement battles, many drugs are being pushed out of the priority list and disappearing. As a result, patients are losing timely access to essential medicines.” He added, “Companies tend to concentrate reimbursement strategies only on flagship products. Alongside government efforts, the industry must itself prioritize expanding patient access.” Specific improvement tasks were also proposed. Tae-Kyung Kim, a specialist at Yoon&Yang criticized the practice of cost-effectiveness-centric evaluations and stressed the need for new approaches. Kim noted, “It's a positive change that various values beyond cost-effectiveness are now reflected in evaluations, leading to higher reimbursement rates for new drugs that would have struggled to gain listing in the past. However, in practice, rather than recognizing the value of new drugs, the structure has solidified into one where the government reduces uncertainty through risk-sharing agreements (RSAs).” “The transfer of uncertainty via risk-sharing agreements is effectively resulting in ‘self-pay by pharmaceutical companies. Health technology assessments must become more flexible to reflect realistic real-world treatment patterns.” Moderator Eui-Kyung Lee, Professor at Sungkyunkwan University’s College of Pharmacy, said, “Post-COVID-19, two values—patient safety and speed—have simultaneously come to the fore. It is essential to find a balance between health insurance finances, timeliness, and safety, while adopting a more multi-dimensional and flexible approach for evaluating the value of innovative medicines.”
- Company
- Xeomin marks 20 years since launch…'pure toxin'
- by Hwang, byoung woo Sep 25, 2025 06:10am
- Celebrating the 20th anniversary of Xeomin's global launch, Merz Aesthetics is emphasizing the importance of 'pure toxin' for managing botulinum toxin resistance. The company explains that in an era of repeated procedures, a highly purified toxin can inhibit antibody formation and become as a sustainable treatment option. Merz Aesthetics Korea held a press conference on September 24 to commemorate the 20th global anniversary of its botulinum toxin type A product, Xeomin, highlighting its scientific value and global leadership. Professor Michael Martin (Retired from Justus Liebig University, Germany)Firstly, Professor Michael Martin (Retired from Justus Liebig University, Germany), an immunology expert from Germany, said that in the global botulinum toxin market, the issue of resistance is no longer optional but a necessary management task. Professor Martin explained, "Only a highly purified toxin free of complexing proteins, inactive neurotoxins, and other impurities can be defined as a true pure toxin. While there are many products on the market, it is challenging to meet all these criteria." He emphasized the importance of pure toxins, citing the consensus of the ASCEND (Aesthetic Council for Ethical use of Neurotoxin Delivery) panel, which includes experts from around the world. The ASCEND panel was formed to systematically review literature and share clinical cases to establish a better standard for botulinum toxin use, as resistance has become an issue with its widespread use in aesthetics beyond therapeutic purposes. Professor Martin said, "To ensure the safe use of botulinum toxin, the key criteria for preventing resistance are to select a highly purified product with a low risk of resistance and to use the minimum effective dose at appropriate intervals." Dr. Juergen Frevert (Merz Pharmaceuticals Consultant)Dr. Juergen Frevert (Merz Pharmaceuticals Consultant), the developer of Xeomin, mentioned that the product is manufactured using Merz's stringent purification technology and a biotechnological production process that is approved by the U.S. Food and Drug Administration (FDA). Dr. Frevert said, "Merz's proprietary purification technology removes complexing proteins that can trigger antibody formation. By using only the active neurotoxin (150kDa) with approved excipients like human serum albumin (HSA) and sucrose, it minimizes the risk of resistance." He added, "In the global botulinum toxin market, pure toxin and resistance prevention are emerging as key keywords. Patients are significantly considering safety, long-term efficacy, and the ease of repeated procedures," and concluded, "Based on scientific evidence, Xeomin provides differentiated value that meets these patient expectations." Xeomin, Strengthening market leadership and ESG commitment In the subsequent presentation, Managing Director So Young Kim of Merz Aesthetics Korea's Brand Marketing Department shared Xeomin's 20-year journey and achievements under the theme of "Xeomin Global Heritage and Leadership." Kim said, "Since its approval by the European Medicines Agency (EMA) in 2005, Xeomin has been approved in 81 countries worldwide, with over 35 million vials supplied cumulatively. In Korea, since its launch in 2009, it has ranked first in import performance for six consecutive years, starting in 2018." Kim added, "Xeomin is a pure toxin that leads the botulinum toxin market and has built a strong trust with medical professionals and patients for a long time since its launch in 2009," and highlighted, "With its room-temperature storage approval in 2023, it contributes to long-term environmental protection by reducing the need for coolants and energy used for refrigeration. We also plan to introduce a new eco-friendly product package." Su Yeon Yu, CEO of Merz Aesthetics Korea, added, "Xeomin has been able to achieve significant results in the global and Korean market over the past 20 years, thanks to the trust of medical professionals and consumers," and concluded, "Merz will continue to meet the expectations of patients and medical professionals through innovative, science-based solutions, while leading the right future of medical aesthetics through ESG management and social responsibility.